Current Pediatric Research
International Journal of Pediatrics
Reduction of paediatric emergence agitation after adenotonsillectomy with nalbuphine.
1Department of Paediatric Anaesthesiology and Critical Care, University Hospital Brno and Faculty of Medicine, Masaryk University Brno, Czech Republic.
2Department of Mathematics and Statistics, Faculty of Science, Masaryk University Brno, Czech Republic.
3Department of Pathological Physiology, Faculty of Medicine, Masaryk University Brno, Czech Republic.
- Corresponding Author:
- Martin Vavřina, MD
Department of Paediatric Anaesthesiology and Critical Care
University Children’s Hospital Brno and Faculty of Medicine
Cernopolni 9, Brno 61300, Czech Republic
Tel: +420777246158
E-mail: vavrina.martin@fnbrno.cz
Accepted date: July 14, 2017
Background and aim: The aim of this prospective, observational study was to evaluate the potential benefit of nalbuphine in paediatric adenotonsillectomy in comparison to alfentanil in the terms of the emergence delirium and other procedural adverse events. Methods: Monitored adverse events were tachycardia, dyspnoea, hypotension, hypertension and emergence agitation according to the adapted Watcha scale. All eligible patients were given oral premedication and general anaesthesia was induced using inhalational or intravenous route. Patients were given nalbuphine (0.1-0.2 mg.kg-1) or alfentanil (10-15 ug.kg-1) and had all requested data recorded in the study form (age, study group, metamizole usage, body weight, total dose of nalbuphine or alfentanil, ASA, studied parameters). Results: Total of 122 patients were enrolled for this study, 8 patients were excluded because of incomplete study form. This resulted in study population of 114 patients. All patients were ASA I–II. No differences in age or body weight were observed. Emergence agitation was significantly (p=0.024) more frequent in the Alfentanil group (39.66%) than in Nalbuphine group (19.64%). Tachycardia was significantly more frequent in younger patients. Dyspnoea was significantly dose-dependent and more frequent in lower dosages (p=0.045). Hypertension was more frequent in patients with higher grade of agitation, but statistically significant only in the Alfentanil group (p=0.044). Conclusion: Nalbuphine in the setting of paediatric adenotonsillectomy makes a good alternative to short acting opioid and postoperative analgesia using NSAID or other nonopioid analgesics. Our results show that nalbuphine provides less emergence agitation and therefore provides a patient better early postoperative outcome.
Keywords
Anaesthesia, Opioid analgesics, Volatile anaesthetics, Sevoflurane, Child, Emergence delirium.
Introduction
Emergence agitation is a common problem in paediatric anaesthesia, which is even more expressed when using sevoflurane induction and maintenance, especially in paediatric patients undergoing adenotonsillectomy. In our department we have been traditionally using alfentanil in the dose of 10-15 ug.kg-1 after sevoflurane or propofol induction for perioperative analgesia and metamizole in the dose of 15 mg.kg-1 for post-operative analgesia. Alfentanil in the induction phase also facilitates intubation conditions. Some studies recommend to use neuromuscular blockade for intubation in adenotonsillectomy, but this approach was not used, since we are not experiencing as many difficult intubations as published in those studies [1-3].
Agitation after the paediatric adenotonsillectomy also poses higher risk of early postoperative complications as bleeding, stridor and cough. Since we had good experience with nalbuphine for perioperative and postoperative analgesia in patients undergoing small surgical procedures (testicle retention, hernia repair, skin incisions, etc.), we decided to compare its effects in adenotonsillectomy. Both drugs showed reduction in postoperative agitation in many studies, but to our knowledge, there is no study directly comparing those two drugs themselves in this setting [4-6].
Nalbuphine hydrochloride is a synthetic opioid μ-receptor antagonist/κ-receptor agonist with the duration of analgesia of 4-6 h. Its analgesic potency approximately equals to morphine with less effects on cardiovascular and respiratory system, i.e., nalbuphine causes less intensive and less frequent decrease in blood pressure and respiratory depression.
Alfentanil is a fast acting synthetic opioid with the peak onset of effect in 1 min and with the duration of effect of 15-30 min. It also provides good intubation conditions in paediatric patients, which makes it ideal opioid for short surgical procedures requiring endotracheal intubation (or laryngeal mask insertion), but less ideal for early postoperative analgesia.
Metamizole is a pyrazolone derivate and potent nonopioid analgesic used for severe acute and chronic pain relief. The onset of effect is 30-40 min and the duration of effect of 4 h.
Our research is a prospective, observational study of paediatric patients undergoing adenotonsillectomy. The aim of this study was to evaluate the potential benefit of nalbuphine in paediatric adenotonsillectomy in comparison to alfentanil in the terms of the emergence delirium and other procedural adverse events.
Materials and Methods
This study was performed according to the principles of the Declaration of Helsinki and institutional Ethics Committee approved and marked this study as compliant with all applicable laws, principles of good clinical practice, Charter of Fundamental Right and Basic Freedoms, Convention for the Protection of Human Rights and Dignity of the Human Being and above mentioned Declaration of Helsinki. Written informed consent was obtained from parents or legal guardians of all study subjects.
The study lasted for two months. The inclusion criteria for the study were the age of 1 to 18 years and the planned adenotonsillectomy.
The exclusion criteria for the study were the intensive care patient sedated with opioids (sufentanil, fentanyl), patient given continuous or intermittent analgesia with tramadol earlier than 4 hours before the surgery, and the patient with chronic pain therapy with morphine or other opiates (oral route, transdermal patches, etc.). We have selected tachycardia, dyspnoea, hypotension, hypertension and early postoperative agitation as procedural adverse events.
Anaesthesia was given by experienced paediatric anaesthesiologist in the university hospital for children (second largest childhood care centre in the country). Adverse events (tachycardia, dyspnoea, hypotension and hypertension) were evaluated as present or not present (marked as “yes”, resp. “no”) according to the established reference values stated in Table 1 (based on The Harriet Lane Handbook, The Johns Hopkins Hospital, Baltimore, Maryland, USA [7]. Early postoperative agitation was evaluated as grade 1-4, where “0” is the calm patient, “1- 2” is slightly agitated patient without the necessity to use the intravenous sedation and “3-4” is extensively agitated patient with the necessity to use the intravenous sedation. Because of the low incidence of emergence agitation in grades 2-4 in our cohort, we decided to correct the agitation grading as “present” or “not present”. We believed that this adjustment will make the results clear, since there are many different measures used in the studies focusing on emergence agitation. Adverse events (tachycardia, dyspnoea, hypotension and hypertension) were recorded at the end of the procedure after the extubation and emergence agitation was recorded at the time of the patient’s transport to the postop ward.
| Tachycardia | |
|---|---|
| 1 month - 1 year | >130 |
| 2 years - 5 years | >110 |
| 6 years - 12 years | >95 |
| Dyspnoea–Normal Breath Rate | |
| 1 month - 1 year | 30-40 |
| 2 years - 5 years | 20-25 |
| 6 years - 12 years | 15-20 |
| Hypotension–Systolic BP | |
| 1 month - 1 year | <80 |
| 2 years - 5 years | <90 |
| 6 years - 12 years | <100 |
| Hypertension–Systolic BP | |
| 1 month - 1 year | >105 |
| 2 years - 5 years | >110 |
| 6 years - 12 years | >120 |
| Emergence Agitation | |
| 0 | Calm patient |
| 1–2 | Slightly agitated patient without the necessity to use the intravenous sedation |
| 3–4 | Extensively agitated patient with the necessity to use the intravenous sedation |
*Adapted from The Harriet Lane Handbook, The Johns Hopkins Hospital
Table 1: Vital signs reference table*
Eligible patients were examined by an attending anaesthesiologist, who recorded all requested data in the study form (age, therapeutic group, metamizole usage, body weight, total dose of nalbuphine or alfentanil, ASA group (American Association of Anaesthesiology) in the beginning of the procedure and also the studied parameters (tachycardia, dyspnoea, hypotension, hypertension and early postoperative agitation) at the end of the procedure.
All eligible patients were given oral premedication (midazolam and atropine) and general anaesthesia using the inhalational route (40% oxygen+60% nitrous oxide+sevoflurane) or the intravenous route (propofol). Intravenous induction was used in the patients with already established i.v. access or in the patients who were physically and mentally appropriate for the i.v. cannula insertion when fully conscious. All other patients received inhalational induction and i.v. access was established afterwards. Before the intubation patients were given nalbuphine (nalbuphine hydrochloride, Nalbuphin Orpha®, G.L. Pharma, Vienna, Austria) in the dose of 0.1-0.2 mg.kg-1 or alfentanil (alfentanil hydrochloride, Rapifen®, GlaxoSmithKline, Parma, Italy) in the dose of 10-15 ug.kg-1. Patients given alfentanil were given metamizole (Novalgin®, Sanofi-Aventis, Prague, Czech Republic) in the standard recommended dose of 15 mg.kg-1 for postoperative analgesia with the duration of effect of approximately 4 hours. Patients were intubated using cuffed or uncuffed ETT according to our standard procedures. Patients were given alfentanil or nalbuphine based on the decision of attending anaesthesiologist; no directions for group assignment were given by study protocol. We believe that this kind of assortment made each anaesthesiologist to use the drug they are most comfortable with and therefore this step could eliminate the physician’s “impact” on the results.
Statistical Methodology
Statistical analysis was performed using STATISTICA v12 software (Dell StatSoft CR, Prague, Czech Republic). Data were tested for independency of the drug type on each of the adverse event types using Pearson chisquare test and Fisher exact test with the determining level of significance 0.05. Further, results of all adverse event types were tested for independency on age, body weight and dose using Wilcoxon rank-sum test (or Mann- Whitney U test) with the correction for continuity. Logistic regression model was created using the emergence agitation as dependent variable and the body weight and age as continuous independent variables (see further in Results).
Results
We enrolled total of 122 patients for this study, 8 patients were excluded because of incomplete study form. This resulted in study population of 114 patients, 58 in the alfentanil group and 56 in the nalbuphine group. All patients were ASA I–II. There were no cases of failed or difficult intubations in the course of this study (data not shown). There were no differences in both groups in age (nalbuphine group 5.1 ± 1.7 years, alfentanil group 5.7 ± 3.1) or body weight (nalbuphine group 21.3 ± 5.4, alfentanil group 23.7 ± 12). Doses given were in the recommended range (nalbuphine 0.17 ± 0.03 mg.kg-1, alfentanil group 0.012 ± 0.004 mg.kg-1). The incidence of emergence agitation in grades 2-4 was quite low in both groups, so we have changed the grading to “present” (original grading 1-4) or “not present” (original grading 0). Table 2 shows the results of all monitored adverse events in both groups.
| Drug Type | Monitored Adverse Event Total number of patients n=114 |
|---|---|
| Tachycardia Present | |
| Nalbuphine | 45/80.35% |
| Alfentanil | 46/79.31% |
| Dyspnoea Present | |
| Nalbuphine | 2/3.58% |
| Alfentanil | 8/13.80% |
| Hypotension present | |
| Nalbuphine | 3/5.36% |
| Alfentanil | 4/6.90% |
| Hypertension present | |
| Nalbuphine | 16/28.58% |
| Alfentanil | 14/24.14% |
| Agitation present | |
| Nalbuphine | 11/19.64% |
| Alfentanil | 23/39.66% |
Table 2: Monitored adverse events
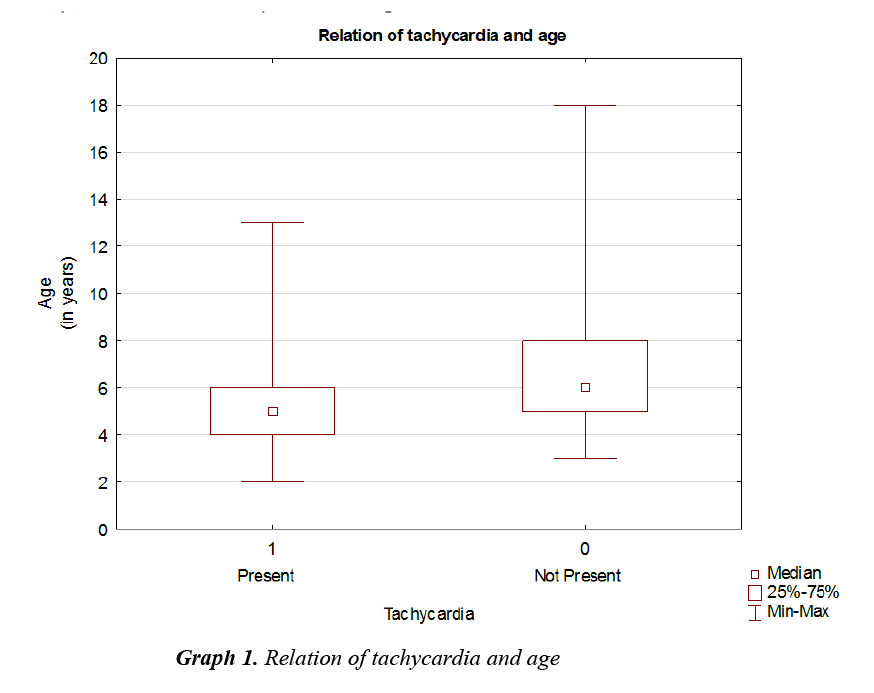
Emergence agitation was significantly more frequent in the Alfentanil group (p=0.024). Tachycardia was significantly age-dependent with higher incidence in younger patients (Graph 1, all patients p=0.001), Nalbuphine group p=0.025, Alfentanil group p=0.028.
Dyspnoea was significantly dose-dependent with higher incidence in lower dosages (all patients p=0.045). But when we look at each group alone, the dyspnoea incidence is not statistically significant (Nalbuphine group p=0.55, Alfentanil group p=0.28).
Hypotension was significantly dose-dependent in Nalbuphine group surprisingly with higher incidence in patients with lower dose (p ≤ 0.001). Hypotension in Alfentanil group was independent on all monitored parameters.
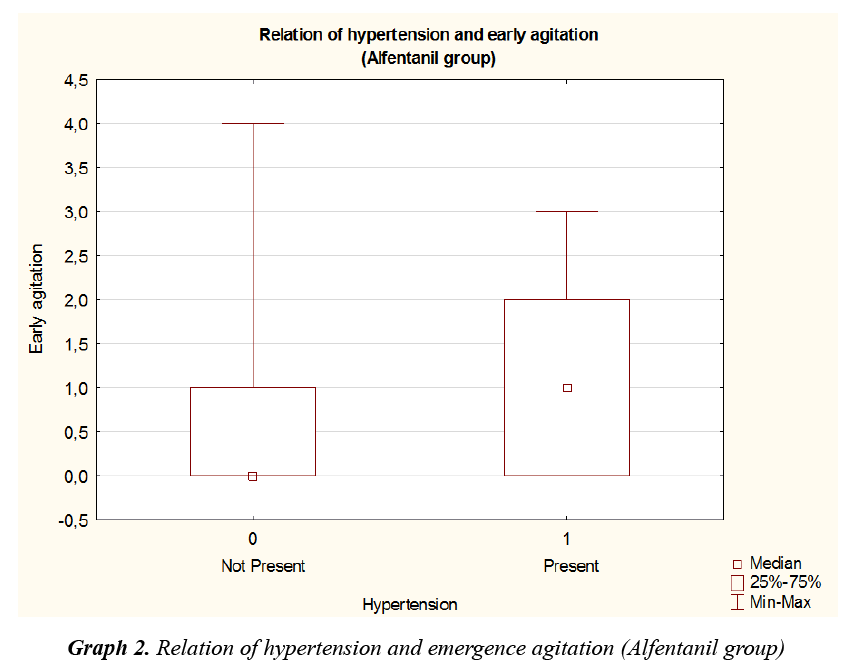
Hypertension was more frequent in patients with original higher grade of agitation (p=0.024), but in the view of both groups separately, the incidence of hypertension with original higher grade of agitation was statistically significant only in the Alfentanil group (Graph 2, Alfentanil group p=0.044, Nalbuphine group p=0.23).
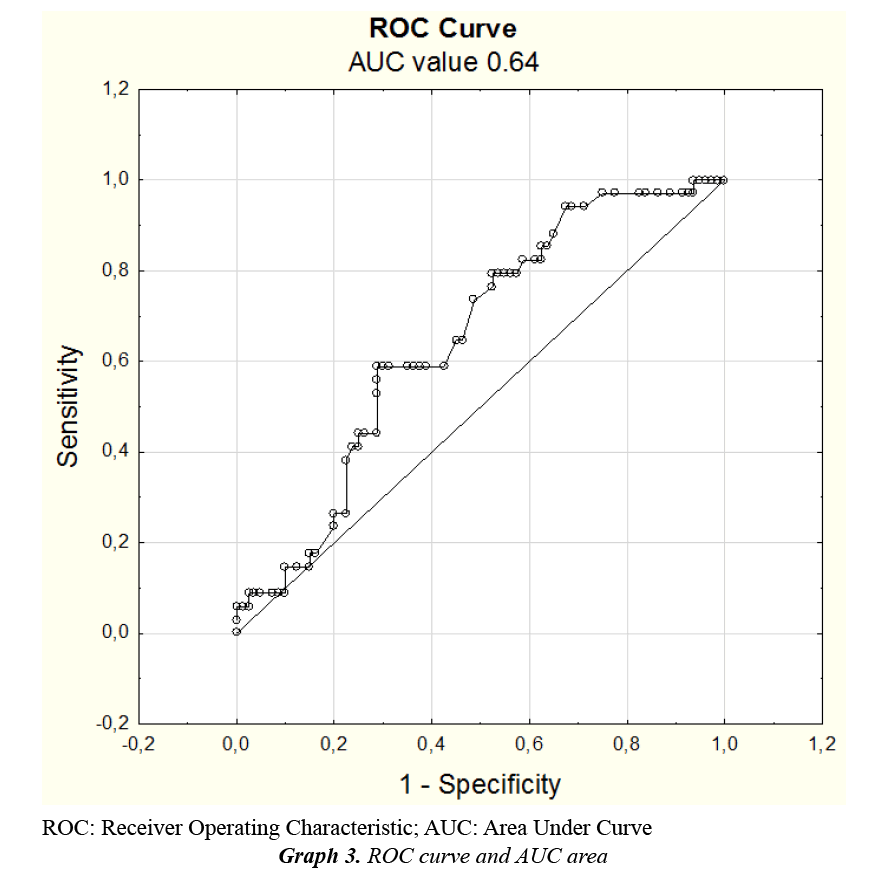
For binary logistic regression modelling we have used emergence agitation as dependent variable, drug type, ASA score, metamizole usage as three independent variables and age, body weight, dose and relative dose as four continuous independent variables. Variables age and body weight were statistically significant on the level of 0.05. So we have created a new model with those two independent variables. Using our model, odds ratio for the age was 0.6 and odds ratio for the body weight was 1.143, which means, that for each year of age the chance for emergence agitation raises 0.6-times and for each kg of body weight the chance raises 1.143-times. Of 34 patients with the emergence agitation, our model correctly assigned 2, i.e., 5.9% and of 80 patients without the emergence agitation model correctly assigned 78, i.e., 97.5%. Thus, total percentage of correct classification was 80/114, i.e., 70.2%. AUC area in our case was 0.64 (Graph 3). Created model is statistically significant and does not contradict our data on one hand, but on the other hand the statistical values, total percentage of correct classification and AUC=0.64 means, that the probability of emergence agitation cannot be satisfyingly explained only by the influence of age and the body weight of the patient.
Discussion
The most important finding of this study is lower incidence of emergence agitation in patients given Nalbuphine. Results for tachycardia are not confounding, since higher heart rate is physiological in smaller children, but on the other hand, chosen reference values can be considered quite low for a child in the postoperative setting. Our other finding, higher incidence of dyspnoea in patients with the lower dosing, can be caused by inadequate analgesia resulting in patient discomfort and therefore in dyspnoea. This finding was statistically significant only for the whole study population and insignificant for each of the study groups. Hypertension results are on the one hand physiological, since patients with higher grade of agitation commonly have higher values of blood pressure, but on the other hand this result was statistically significant only in the Alfentanil group, which further encourages our conclusion, that patients with Alfentanil were more agitated, had higher heart rate and higher blood pressure, which puts together worse early postoperative outcome in paediatric patients after adenotonsillectomy. Very interesting finding is higher incidence of hypotension in Nalbuphine group of patients with lower dose. Hypotension is one of the commonly described adverse reactions to opioids application, to which group Nalbuphine also belongs, but in the terms of overdose, not underdose. We do not have any meaningful explanation for this phenomenon, but this is definitely a result worth further research in the larger group of patients.
Many studies focus on Emergence Agitation (EA) and many procedures were described in order to minimize this effect of sevoflurane. But not only with sevoflurane, emergence agitation was also described when using isoflurane, so this effect cannot be assigned to sevoflurane alone, but to actually used volatiles themselves [8].
One of the procedures to minimize the EA described, and in fact logical, is the usage of premedication in higher dosages. But midazolam, as most widely used premedication drug, failed to be adequately proven in minimizing the incidence of EA [9,10]. Other described options are single dose opioids, mostly sufentanil, fentanyl and remifentanil, at the conclusion of the procedure, which shows good effect in minimizing the EA, but there are also some serious concerns about their side effects such as nausea, vomiting and respiratory depression [11-13]. Alfentanil itself also showed reduction in the incidence of EA, but only in the comparison with sevoflurane monoanesthesia [14]. Usage of additional intravenous anaesthetics, mainly propofol, showed mixed results concerning the EA. Some studies showed no effect in reducing the incidence of EA and some studies proved it to be useful in this indication, but for the price of small increase in recovery time [9,13,15-17]. Best results were marked when using dexmedetomidine, which seems to be very effective in preventing or minimizing the EA, but there is also an increase in time to emergence and extubation and wide usage of dexmedetomidine in our setting is also limited by the price of this drug [9,18- 20]. Our decision to use nalbuphine for comparison with alfentanil in paediatric adenotonsillectomy was based on its good analgesic potential, which is described to be similar with morphine, and poses lower risk of opioid side effects such as nausea, vomiting or respiratory depression [6]. There are also some studies showing good effect in the prevention of EA in paediatric anaesthesia setting [5].
Also we have to note, that most of those studies use Paediatric Anaesthesia Emergence Delirium scale (PAED), but our original agitation grading is an adapted version of Watcha scale [21]. Because of this, there is quite low potential to make any comparison with those studies, but we have used adapted Watcha, since it is quicker and less complicated to complete in the operating room in our setting.
The weakness of this study is relatively small number of patients to make any assumptions generally applicable to whole paediatric population. Other strong weakness is the subjectivity of emergence agitation scoring. As noted, we have used the adapted version of Watcha scale, but any scoring system for emergence agitation cannot fully clear off the bias caused by the subjective measures of each physician [21]. On the other hand, during the statistical analysis we have minimalized this subjectivity by the change of EA grading 0-4 to simply present or not present [22].
Conclusion
In our opinion, Nalbuphine in the setting of paediatric adenotonsillectomy makes a good alternative to short acting opioid and postoperative analgesia using NSAID or other non-opioid analgesics. Our results show that nalbuphine provides less emergence agitation and therefore provides a patient better early postoperative outcome.
Acknowledgement
Authors would like to acknowledge the work of our anaesthesiology nursing staff providing care for study patients and assistance to medical doctors during the cases and also the work of RNDr. Marie Budíková, Dr. who added valuable help with the statistical analysis and the manuscript review.
Disclosures
Ethical approval
Separate committee confirmation: This study was performed according to the principles of the Declaration of Helsinki and institutional Ethics Committee approved and marked this study as compliant with all applicable laws, principles of good clinical practice, Charter of Fundamental Right and Basic Freedoms, Convention for the Protection of Human Rights and Dignity of the Human Being and above mentioned Declaration of Helsinki. Written informed consent was obtained from parents or legal guardians of all study subjects.
IRB contact: Ethics Board of the University Hospital Brno, chairman: PharmDr. Šárka Kozáková, MBA, email: kozakova@mou.cz, mailing address: Ethics Board of the University Hospital Brno, Jihlavská 20, Brno, 62500, Czech Republic.
Study registration
This study was non-interventional comparison of two common and accepted techniques and as such it was not registered in any clinical trial registry.
Source of funding
Research was fully funded from departmental resources. We declare no study sponsor or support.
Conflicts of interest
All authors declare no conflicts of interest.
References
- Kim J, Lee J, Park H, et al. The effect of alfentanil versus ketamine on the intubation condition and hemodynamics with low-dose rocuronium in children. J Anesth 2013; 27: 7-11.
- Kwak HJ, Kim JY, Min SK, et al. Optimal bolus dose of alfentanil for successful tracheal intubation during sevoflurane induction with and without nitrous oxide in children. Br J Anaesth 2010; 104: 628-632.
- Bartolek D, Lajtman Z, Zdravčević-Šakić K, et al. The optimal pediatric induction dose of propofol in combination with reduced-dose rocuronium and alfentanil for day-case tonsillectomy in children. Int J Pediatr Otorhinolaryngol 2007; 71: 1873-1881.
- Krishnan A, Tolhurst-Cleaver CL, Kay B. Controlled comparison of nalbuphine and morphine for post-tonsillectomy pain. Anaesthesia 1985; 40: 1178-1181.
- Dalens BJ, Pinard AM, Létourneau DR, et al. Prevention of emergence agitation after sevoflurane anesthesia for pediatric cerebral magnetic resonance imaging by small doses of ketamine or nalbuphine administered just before discontinuing anesthesia. Anesth Analg 2006; 102: 1056-1061.
- Schnabel A, Reichl SU, Zahn PK, et al. Nalbuphine for postoperative pain treatment in children. Cochrane Database Syst Rev 2014; 7: CD009583.
- Custer JW, Rau RE. The Harriet Lane Handbook. 18th ed. Philadelphia: Mosby/Elsevier. 2009.
- Meyer RR, Münster P, Werner C, et al. Isoflurane is associated with a similar incidence of emergence agitation/delirium as sevoflurane in young children--a randomized controlled study. Paediatr Anaesth 2007; 17: 56-60.
- Costi D, Cyna AM, Ahmed S, et al. Effects of sevoflurane versus other general anaesthesia on emergence agitation in children. Cochrane Database Syst Rev 2014; 9: CD007084.
- Breschan C, Platzer M, Jost R, et al. Midazolam does not reduce emergence delirium after sevoflurane anesthesia in children. Paediatr Anaesth 2007; 17: 347-352.
- Liang P, Zhou C, Ni J, et al. Single-dose sufentanil or fentanyl reduces agitation after sevoflurane anesthesia in children undergoing ophthalmology surgery. Pak J Med Sci 2014; 30: 1059-1063.
- Dong YX, Meng LX, Wang Y, et al. The effect of remifentanil on the incidence of agitation on emergence from sevoflurane anaesthesia in children undergoing adenotonsillectomy. Anaesth Intensive Care 2010; 38: 718-722.
- Kim MS, Moon BE, Kim H, Lee JR. Comparison of propofol and fentanyl administered at the end of anaesthesia for prevention of emergence agitation after sevoflurane anaesthesia in children. Br J Anaesth 2013; 110: 274-280.
- Kim JY, Chang YJ, Lee JY, et al. Post-induction alfentanil reduces sevoflurane-associated emergence agitation in children undergoing an adenotonsillectomy. Acta Anaesthesiol Scand 2009; 53: 678-681.
- Lee CJ, Lee SE, Oh MK, et al. The effect of propofol on emergence agitation in children receiving sevoflurane for adenotonsillectomy. Korean J Anesthesiol 2010; 59: 75-81.
- Pieters BJ, Penn E, Nicklaus P, et al. Emergence delirium and postoperative pain in children undergoing adenotonsillectomy: A comparison of propofol vs sevoflurane anesthesia. Paediatr Anaesth 2010; 20: 944-950.
- Costi D, Ellwood J, Wallace A, et al. Transition to propofol after sevoflurane anesthesia to prevent emergence agitation: A randomized controlled trial. Paediatr Anaesth 2015; 25: 517-523.
- Patel A, Davidson M, Tran MCJ, et al. Dexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesth Analg 2010; 111: 1004-1010.
- Dahmani S, Delivet H, Hilly J. Emergence delirium in children: an update. Curr Opin Anaesthesiol 2014; 27: 309-315
- Guler G, Akin A, Tosun Z, et al. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth 2005; 15: 762-766.
- Reduque LL, Verghese ST. Paediatric emergence delirium. Contin Educ Anaesth Crit Care Pain 2013; 13: 39-41.
- http://www.statsoft.com