Current Pediatric Research
International Journal of Pediatrics
Pyloric stenosis of infancy, hyperacidity and Occam's razor.
I. M. Rogers*
Department of Pediatrics, University of Sunderland, Sunderland, United Kingdom
- Corresponding Author:
- I. M. Rogers
Department of Paediatrics,
University of Sunderland,
Sunderland,
United Kingdom
E-mail: irogers2000@hotmail.com
Received: 18 August, 2020, Manuscript No. AAJCP-24-17650; Editor assigned: 21 August, 2020, Pre QC No. AAJCP-24-17650 (PQ); Reviewed: 04 September, 2020, QC No. AAJCP-24-17650; Revised: 01 October, 2024, Manuscript No. AAJCP-24-17650 (R); Published: 29 October, 2024, DOI: 10.35841/0971-9032.28.10.2327-2334.
Despite the multiple clinical clues which these infants give us and despite the passage of 300 years since its first discovery, we remain still quite ignorant of the pathogenesis. The essential given clinical characteristics regularly documented include the 5/1 male sex ratio/preponderance of first born infants presentation when the tumour is acquired at around 4 weeks of age spontaneous self-cure if the infant survives for several weeks with medical treatment problems with gastric hyperacidity in later life dangerous hypokalaemic alkalosis from loss of acid before the obstruction is relieved.
Keywords
Neonatal hyperacidity, Pathogenesis, Pyloric stenosis of infancy (PS).
Introduction
Curiously the first recorded attempt at a pathogenesis by Freund in 1903 declared that a hydrochloric acid content in excess of normal was a causative factor in spasticity of the pylorus. This proposal seems gradually to have been forgotten and the undoubted acidity of the gastric contents, was presumed to be due to secondary retention of acid. In retrospect an unwarranted assumption. The following facts have now been established [1].
Description
Acid entering the adult duodenum is a potent cause of pyloric sphincter contraction. The histology of the typical pyloric tumour is consistent with hypertrophy due to repeated contraction. The gastro-intestinal trophic hormone gastrin is greatly elevated in normal development in the first few weeks of life. Hence sphincter work hypertrophy in infants with hyperacidity will be especially vigorous at this time. The pyloric sphincter contracts most frequently after meals when it presents a closed sphincter to post-prandial gastric peristalsis thereby mixing up food with gastric secretions. The PS baby is classically eager for food, presumably is likely to be fed more often and will be especially susceptible to sphincter hypertrophy [2].
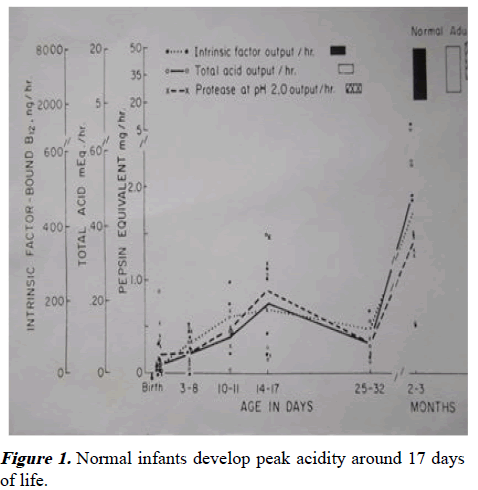
PS infants secrete more acid than normal infants and that this phenomenon continues even when pyloric obstruction is relieved. This truth is confirmed by the observation that when vomiting infants develop hypochloraemic alkalosis, PS is invariably present. There is a peak in acid secretion in normal development at around 17 days of age-a time which would comfortably be consistent with the age of presentation of PS [3].
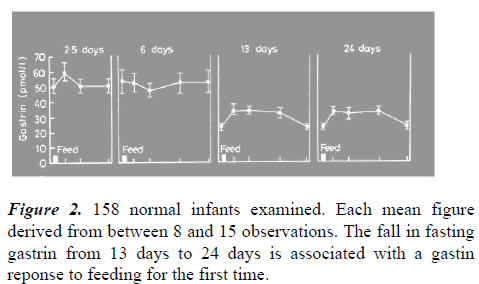
It has been proposed that there is an immature negative feedback mechanism between gastrin and antral acidity from birth which takes some weeks to mature. Evidence for this is the high fasting gastrins in the first weeks of age (with no post-feed increase) and a lowered fasting gastrin after this with a postfeed response. Mandatory maximal gastrin secretion initially followed by a fall in fasting gastrin and the development of a post-feed gastrin response, would be the expected result if the negative feed-back system took time to mature. A progressive rise in acidity which peaks when the feed-back is mature would similarly be expected. Both phenomena have been observed (Figures 1 and 2) [4].
Indeed it is logical to suppose that the initially ineffective negative feed-back is itself sufficient to cause gastrin and acid to rise progressively to a peak and then fall when feed-back is established. The addition of IV Cimetidine to the treatment of PS babies pre-operatively restores the acid-base status so quickly, that same day surgery is possible. When the sphincter thickness is 4 mm or less. IV cimetidine also produces a long lasting cure in 16 out of 17 babies. The regular need for nasogastric feeding in premature babies has made it ethically possible to measure their acidity. Premature male infants have been shown to have greater acidity than females. It has been long known that male adults similarly outperform adult females where acid secretion is concerned [5].
The male preponderance of PS. exactly parallels the sex-ratio of adult duodenal ulcer patients, a condition known to be due to too much acid. When developmental peak acidity occurs in babies born with inherited hyperacidity they will be especially liable to develop frequent sphincter contractions and work hypertrophy. The celebrated dog model for pyloric stenosis conceived by Professor John Dodge produced pyloric stenosis in puppy dogs born to bitches treated with parenteral pentagastrin just before birth. It has been established that gastrin crosses the canine placenta and causes gastric acid secretion. Even more puppies developed PS when the injections were given to them directly.
Conclusion
Acid provokes sphincter contraction. Repeated sphincter contraction aided by high gastrin levels produces sphincter hypertrophy. There is peak acidity in normal development just before the time that PS infants present. Why should be look further afield for an alternative cause? The stomach secretes acid and pepsin and the sphincter intermittently contracts to ensure feed mixing. Inappropriately frequent feeding coupled with inherited hyperacidity combine to to produce the potentially killing condition of PS. It has not escaped attention that this mechanism of ensuring relatively high acidity for the first few week of life in normal development, ought to protect against early enteric infections. The mechanism may have developed and been maintained by evolutionary pressures. PS of infancy may be one of the few disadvantages of this phenomenon. Reproduced with permission from BMJ publishing. The fasting gastrin at 2.5 to 6 days is 7 times greater than the fasting adult level. From development of gut hormone responses to feeding in neonates.
References
- Agunod M, Yamaguchi N, Lopez R, et al. Correlative study of hydrochloric acid, pepsin and intrinsic factor secretion in newborns and infants. Am J Dig Dis1969; 14: 400-414.
[Crossref] [Google Scholar] [PubMed]
- Banieghbal B. Rapid correction of metabolic alkalosis in hypertrophic pyloric stenosis with intravenous cimetidine: Preliminary results. Pediatr Surg Int 2009; 25: 269-271.
[Crossref] [Google Scholar] [PubMed]
- Ames MD. Gastric acidity in the first ten days of life of the prematurely born baby. Am J Dis Child 1960; 100: 252-256.
[Crossref] [Google Scholar] [PubMed]
- Dodge JA, Karim AA. Induction of pyloric hypertrophy by pentagastrin. An animal model for infantile hypertrophic pyloric stenosis. Gut 1976; 17: 280-284.
[Crossref] [Google Scholar] [PubMed]
- Rogers IM. Pyloric stenosis of Infancy, primary hyperacidity and occam’s razor. Med Hypotheses 2020; 145: 110325.
[Crossref] [Google Scholar] [PubMed]