Current Pediatric Research
International Journal of Pediatrics
Neurofibromatosis and type-1 diabetes in a seven-year-old child: A rare combination.
Department of Pediatric Endocrinology, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia
- Corresponding Author:
- Abdulmoein Eid Al-Agha
Professor, Pediatric Endocrinology, Pediatric Department
King Abdulaziz University Hospital, P.O. Box 80215, Jeddah 21589, Saudi Arabia
Tel: + 966 2 640 3841; + 966 2 6408353
Fax: + 966 2 640 3841; + 966 2 6408353
E-mail: aagha@kau.edu.sa
Accepted date: June 20th, 2018
Neurofibromatosis type 1 (NF1) can occur along with other autoimmune diseases. We report the case of a child with coexisting neurofibromatosis and type 1 diabetes mellitus. A seven-year-old Yemeni girl, who was diagnosed with neurofibromatosis type 1 at birth, presented at the age of two months with her first episode of diabetic ketoacidosis. Further lab investigations during her follow-up sessions showed a decreased level of growth hormone. Abnormal neurofibromin production is the main pathophysiology of neurofibromatosis that contributes to an increased susceptibility to tumors and other coexisting autoimmune diseases; this requires further discussion. This is, to the best of our knowledge, the third case reported to have this rare combination of a de novo mutation causing NF1 and an autoimmune disease, which is type 1 diabetes mellitus.
Keywords
Neurofibromatosis, Type 1 diabetes, Children, Association, Autoimmune.
Introduction
Neurofibromatosis type 1 (NF1) is a multisystem disease of autosomal dominant inheritance, with an incidence of 1 in 3000 people worldwide [1]. It consists of cutaneous findings, most notably café-au-lait spots and axillary freckling, skeletal dysplasia, and the growth of benign and malignant neural crest tumors, and most commonly benign neurofibromas [1]. It occurs due to genetic mutation on the NF1 gene in chromosome 17q11.2 that codes for a tumor suppressor protein called neurofibromin. However, very occasionally, NF1 is also associated with other autoimmune diseases including systemic lupus erythematosus, autoimmune thyroiditis, vitiligo, IgA nephropathy, multiple sclerosis, and an exceedingly rare association with diabetes mellitus type 1 [2,3]. In addition to autoimmune disease association, neurofibromatosis could also manifest in rare neuroendocrine tumors like somatostatinomas and insulinomas [4]. To the best of our knowledge, only two patients have been reported to have both NF1 and type 1 diabetes mellitus until now [4,5].
The United Nations regards diabetes as the most common chronic metabolic disease diagnosed in children and adolescents [6]. Developing countries have reported the highest rate of incidence of type 1 diabetes in children worldwide, and it has increased from 3% to 5% since the 1960s [7]. We report a rare case of co-existing NF1 and type 1 diabetes mellitus in a 7-year-old child.
Case Report
A 7-year-old Yemeni girl presented to our hospital with an episode of diabetic ketoacidosis that presented with vomiting and abdominal pain and was resolved with the appropriate treatment. She had been diagnosed with NF1 at birth based on the presence of multiple painless cafe au lait spots (measuring 1-7 mm) as well as axillary and inguinal freckles. She was diagnosed with type 1 diabetes mellitus at 2 months of age. She has been on treatment with insulin isophane and soluble insulin for her diabetes since then; her current treatment regime is insulin glargine. She has no complications such as retinopathy, nephropathy, or neuropathy. None of her relatives had either condition, despite the relative consanguinity between her father and the mother. She did not have neurofibroma, plexiform neurofibroma, optic nerve glioma, long bone abnormalities, hearing loss, visual deficit, or balance problems. Her development matched her chronological age with respect to gross motor, fine motor, visual, speech, and social skills.
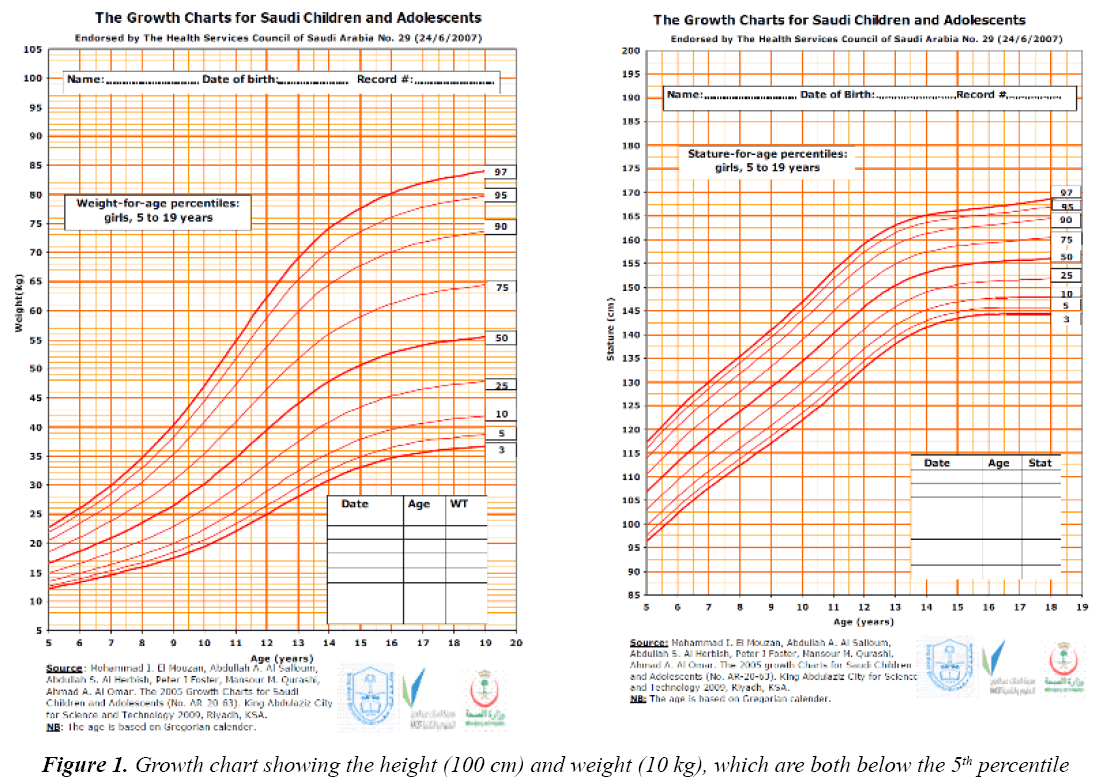
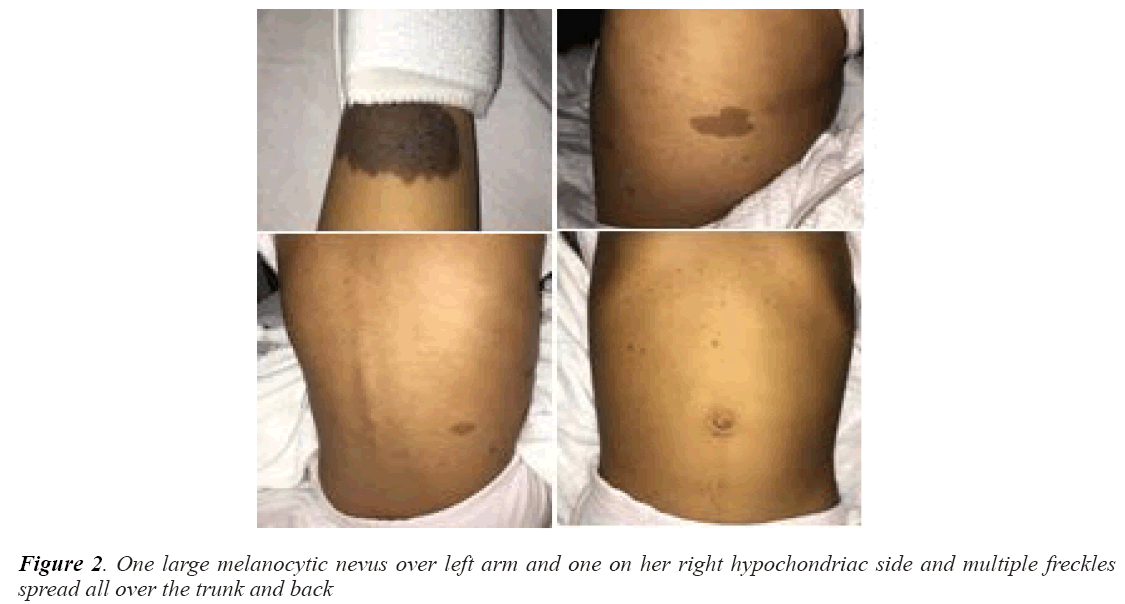
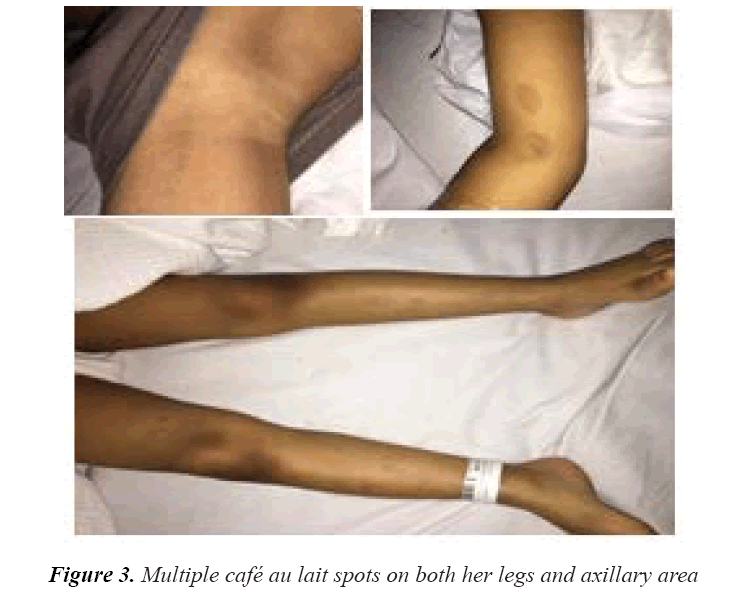
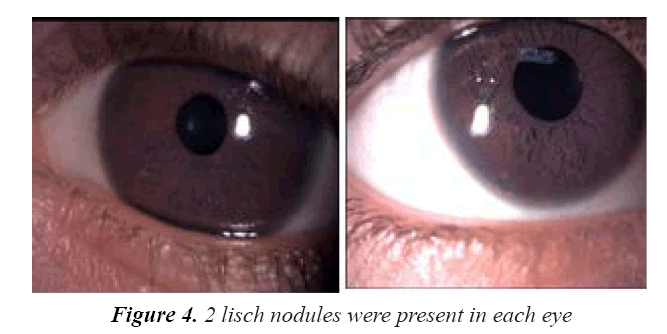
On examination, her vitals were stable. Her growth parameters were as follows: weight, 10 kg and height, 100 cm (Figure 1). She had multiple cafe au lait spots, one large melanocytic nevus over her left arm and one on her right hypochondriac side, along with freckles spread all over the trunk and back (Figure 2) including both her legs and axillary area (Figure 3). The patient was fully oriented and had normal reflexes, cranial nerve, cerebellar, motor, and sensory nerve function. The baseline ophthalmological evaluation showed that 2 Lisch nodules were present in each eye (Figure 4); however, the normal optic disc, macula, and retinal blood vessels were normal and her vision was 20/20. The results of the investigations are shown in Table 1. The MRI findings were unremarkable and showed no tumors (Figure 5). At present, the patient's diabetes is well controlled and no modifications have been made in her treatment regime. She is following up regularly.
| Investigation | Value result | Normal range |
| Growth hormone | 1.61 | >10 ng/mL |
| Random glucose | 27.6 | 3.9-6.7 mmol/L |
| HbA1C | 12.3 | 4.2-6.3 % |
| TSH (Thyroid Stimulating Hormone) | 2.83 | 0.27-4.2 ulU/L |
| Free T4 | 15.5 | 12-22 pmol/L |
| Total protein | 80 | 64-82 gm/L |
| Albumin | 36 | 40.2-47.6 gm/L |
| Alanine aminotransferase | 20 | 12-78 U/L |
| Aspartate aminotransferase | 17 | 15-37 U/L |
| Alkaline phosphatase | 175 | 156-369 U/L |
| Gamma GT (Glutamyl Transpeptidase) | 8 | 5-85 U/L |
| BUN (Blood Urea Nitrogen) | 10.8 | 2.5-6.4 mmol/L |
| Serum Creatinine | 64 | 53-115 umol/L |
| Urine creatinine | 1660 | 53000-2200 umol/L |
| Calcium | 2.20 | 2.12-2.52 mmol/L |
| Phosphate | 1.97 | 0.81-1.58 mmol/L |
| Vitamin D | 28 | 75-250 nmol/L |
Table 1: Biochemical investigations report
Discussion
NF1, previously called von Recklinghausen’s disease, is a common autosomal dominant inherited disease with a reported incidence of 1 in 3000 people worldwide [1]. It is a genetic germ-line–inactivating mutation on the Nf1 gene in chromosome 17q11.2 that codes for neurofibromin, which acts as a tumor suppressor protein [8]. This usually explains why there is an increased susceptibility to neoplasms of neural crest origin, including the neurofibromas [9]. Neurofibromin also suppresses the expression of the Fas ligand, preventing apoptosis of CD4+ T-cells, which may contribute to the development of autoimmune conditions due to abnormal neurofibromin production, while decreased T cell apoptosis may be an underlying cause for the development of the autoimmune diseases mentioned earlier [1].
There are seven diagnostic criteria for NF1: more than two neurofibromas, freckling of the axilla (Crowe’s sign) and the groin, skeletal abnormalities such as cortical thinning of the long bones of the body or sphenoid dysplasia, six or more café-au-lait lesions that have a maximum diameter of 5 mm in the pediatric age group and 15 mm in adults, two Lisch nodules or more (hamartomas of the iris), optic glioma/CNS tumors, or a first-degree family member with NF1. If any two of these seven criteria are noted, NF1 is diagnosed [10,11].
Although the three main characteristics (Lisch nodules, café au lait spots, and neurofibromas) occur in more than 90% of NF1 patients at puberty, other characteristics are present in a few cases as well, such as learning disabilities, scoliosis, macrocephaly, pseudarthrosis, seizures, malignancies, and short stature [8,12-15]. In our patient, she had constant fatigue with a mild learning disability. While the main cause for short stature in children with NF1 has not been understood completely, some studies have suggested a primary effect of the Nf1 gene regarding inactivation of somatic and bone growth. In previous studies, genetically engineered mice without Nf1 expression in bone tissue were seen to have skeletal irregularities [16,17]; in other genetically engineered mice without Nf1 expression in the nervous system, the neurons were seen to have decreased hypothalamic-pituitary axis action and insulin-like growth factor-I (IGF-1) levels [18]. Short stature could also be the cause of NF1-associated co-morbidities, such as precocious puberty [19], unlike in our case. Lastly, growth hormone deficiency is also a cause of short stature [20]. The normal peak level of growth hormone is 10 ng/ml; in our patient, the growth hormone stimulation test (clonidine and glucagon pharmacological stimulation) confirmed that growth hormone deficiency (1.61 ng/ml) was the cause of short stature. Approximately 50% of NF1 cases are familial (inherited). The rest result from de novo (sporadic) mutations; these occur mostly in paternal chromosomes, and the chance of de novo NF1 increases with advanced paternal age [1]; in our patient, there was no family history and we suspect that her diagnosis was de novo, as we were unable to perform genetic testing on her father.
International research (DIAMOND and EURODIAB) shows a growing worldwide trend in type 1 diabetes in children under 18 years. Additionally, there are significant differences in the incidence of the disease among different countries, with the lowest rates recorded in China and Venezuela (1 per 100 000 people annually) and the highest in Finland and Sardinia (37 per 100 000 people annually) [6]. The primary pathology involves autoimmune pancreatic beta cell destruction and organ damage, which occur due to various causes and bring about absolute insulin deficiency [6]. The association between diabetes mellitus and NF1 might be explained by the appearance of pancreatic and duodenal tumors like somatostatinomas and insulinomas [21]. Somatostatinomas are uncommon neoplasms that ordinarily emerge in the pancreas or duodenum. The clinical signs of somatostatinoma (diabetes mellitus, cholelithiasis, and steatorrhea) result from the reduced production of other gastrointestinal hormones due to high somatostatin levels [22]. Insulinomas are the most widely recognized endocrine neoplasm. They are insulin-emitting tumors of pancreatic source that cause hypoglycemia; they do not have any specific age or sex predominance. Approximately 90% are singular, >90% occur at intrapancreatic destinations, and 90% are <2 cm in diameter [23].
If insulinoma is diagnosed, genetic testing is often performed for mutations of the MEN1 gene. Patients with this mutation should be evaluated for pituitary, parathyroid, and other pancreatic tumors [22].
To the best of our knowledge, only two previous cases have been recorded of the NF1 comorbid with type 1 diabetes mellitus. However, both patients were boys aged between 9-15 years.
Conclusion
There is an elevated incidence of NF1 associated with other autoimmune diseases. Furthermore, other autoimmune diseases could be suspected in a child with multiple café au lait lesions; this increases the need for autoimmune disease screening.
References
- Ozhan B, Ozguven AA, Ersoy B. Neurofibromatosis type 1 and diabetes mellitus: an unusual association. Case Rep Endocrinol 2013; 2013: 689107.
- Kamoun M, Charfi N, Rekik N, et al. Neurofibromatosis and type 1 diabetes mellitus: An unusual association. Diabet Med 2009; 26: 1180-1181.
- Abbas AK. Diseases caused by immune responses: Hypersensitivity and autoimmunity. Cellular and Molecular Immunology. 8th edn. Elsevier Health Sciences 2000.
- Williams VC, Lucas J, Babcock MA, et al. Neurofibromatosis type 1 revisited. Pediatrics 2009; 123: 124-133.
- Shen MH, Harper PS, Upadhyaya M. Molecular genetics of neurofibromatosis type 1 (NF1). J Med Genet 1996; 33: 2-17.
- Maertens O, Brems H, Vandesompele J, et al. Comprehensive NF1 screening on cultured Schwann cells from neurofibromas. Hum Mutat 2006; 27: 1030-1040.
- Nabi J. Neurofibromatosis type 1 associated with Hashimoto’s thyroiditis: Coincidence or possible link. Case Rep Neurol Med 2013; 910656.
- Chistiakov DA. Immunogenetics of Hashimoto’s thyroiditis. J Autoimmune Dis 2005; 2: 1.
- Nesi G, Marcucci T, Rubio CA, et al. Somatostatinoma: Clinico‐pathological features of three cases and literature reviewed. J Gastroenterol Hepatol 2008; 23: 521526.
- Krzewska A, Ben-Skowronek I. Effect of associated autoimmune diseases on type 1 diabetes mellitus incidence and metabolic control in children and adolescents. Biomed Res Int. 2016: 6219730.
- Friedman JM. Neurofibromatosis: Phenotype, natural history, and pathogenesis. Johns Hopkins University Press. 1999.
- Riccardi VM. Cutaneous manifestation of neurofibromatosis: Cellular interaction, pigmentation, and mast cells. Birth Defects Orig Artic Ser 1981; 17: 129-145.
- Huson SM, Compston DA, Clark P, et al. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. I. Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J Med Genet 1989; 26: 704-711.
- Huson SM, Compston DA, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. II. Guidelines for genetic counselling. J Med Genet 1989; 26: 712-721.
- Zhang W, Rhodes SD, Zhao L, et al. Primary osteopathy of vertebrae in a neurofibromatosis type 1 murine model. Bone 2011; 48: 1378-1387.
- Wang W, Nyman JS, Ono K, et al. Mice lacking Nf1 in osteochondroprogenitor cells display skeletal dysplasia similar to patients with neurofibromatosis type I. Hum Mol Genet 2011; 20: 3910-3924.
- Hegedus B, Yeh TH, Lee DY, et al. Neurofibromin regulates somatic growth through the hypothalamic–pituitary axis. Hum Mol Genet 2008; 17: 2956-2966.
- Zacharin M. Precocious puberty in two children with neurofibromatosis type I in the absence of optic chiasmal glioma. J Pediatr 1997; 130: 155-157.
- Vassilopoulou-Sellin R, Klein MJ, Slopis JK. Growth hormone deficiency in children with neurofibromatosis type 1 without suprasellar lesions. Pediatr Neurol 2000; 22: 355-358.
- Soucy EA, Van Oppen D, Nejedly NL, et al. Height assessments in children with neurofibromatosis type 1. J Child Neurol 2013; 28: 303-307.
- Okabayashi T, Shima Y, Sumiyoshi T, et al. Diagnosis and management of insulinoma. World J Gastroenterol 2013; 19: 829-837.
- Padidela R, Fiest M, Arya V, et al. Insulinoma in childhood: clinical, radiological, molecular and histological aspects of nine patients. Eur J Endocrinol 2014; 170: 741-747.
- Jarosz-Chobot P, Polanska J, Szadkowska A, et al. Rapid increase in the incidence of type 1 diabetes in Polish children from 1989 to 2004, and predictions for 2010 to 2025. Diabetologia 2011; 54: 508-515.