Current Pediatric Research
International Journal of Pediatrics
Management of severe asthma in pediatric patients by an interdisciplinary team
Teijeiro A*, Vieyra RE, Arteaga AE, Ermoli M, Saenz G, Bustos C, Raiden MG, Yanez A
Department of Respiratory, Children's Hospital, Córdoba, Argentina
- Corresponding Author:
- Teijeiro A Department of Respiratory, Children's Hospital, Córdoba, Argentina E-mail: ateijeirom@gmail.com
Received: 26 December, 2022, Manuscript No. AAJCP-22-47978; Editor assigned: 28 December, 2022, Pre QC No. AAJCP-22-47978 (PQ); Reviewed: 05 January, 2023, QC No. AAJCP-22-47978; Revised: 20 January, 2023, Manuscript No. AAJCP-22-47978 (R); Published: 31 January, 2023, DOI:10.35841/0971-9032.27.01.1752-1758.
Introduction: The implementation of interdisciplinary care strategies for the follow-up of patients with asthma has proven to be very effective in improving evolution of these children.
Objectives: To identify, through an interdisciplinary team, the differences in characteristics and management between patients with Severe Difficult to Treat Asthma (SDCA) and Severe Treatment Resistant Asthma (STRA). A secondary objective was reducing rates of hospitalizations and visits to the emergency ward, as well as exacerbations.
Methods: Analysis of case series of patients with UCSA diagnosis admitted to the pneumonology service of the pediatric hospital of Córdoba, aged between 5 and 15 year-old, with a 6 months followup.
Results: 23 patients entered the study, 47.82% were defined as SDCA and 52.17% STRA. During the first 6 months of the program, hospitalization rate was only 4% (p=0.001). Number of visits to the emergency room decreased to 39.13% (p=0.003). Regarding the inhalation technique, 73.9% (n=17) presented improvement (p=0.0001). Only 13% (n=3) of the patients continued with ACT score <20 (p=0.0001). We found differences in the use of rescue medication (p=0.003) and greater attendance at emergency rooms (p=0.005) during the 6 months evaluation process in the treatmentresistant asthmatic patients group.
Conclusion: Implementing this type of interdisciplinary program was associated with a marked improvement in all modifiable variables of asthma in patients with SDCA, leading to a better management of UCSA patients.
Keywords
Pediatric asthma, Interdisciplinary team, Uncontrolled severe asthma, Severe asthma program.
Introduction
Asthma is an important global healthcare problem. According to the 2014 Global Asthma report from the World Health Organization (WHO), 334 million patients are affected worldwide. Asthma burden, in terms of disability and premature death, is higher in preadolescent patients (aged 10-14 years) [1].
In Argentina, asthma prevalence among children and adolescents has been analyzed in the International Study of Asthma and Allergies in Childhood (ISAAC) research program. According to ISSAC phase-I data, prevalence of wheezing among children aged 6-7 and 13-14 years was 16% and 11.5% in Cordoba, respectively. In ISAAC phase-III, prevalence rate among adolescents aged 13-14 years was 13.6%. The exact proportion of pediatric patients with Severe Asthma (SA) remains unknown. Even though SA represents only 5% to 7% of asthma cases in previous research, this subgroup of patients are considered highly vulnerable, with a relevant socioeconomic and psychological burden [1-3]. In the Asthma Insights and Reality in Latin America (AIRLA) survey, the rate of a combined endpoint regarding inadequate asthma control (use of emergency care in past 12 months: hospitalization, emergency service visit and unscheduled emergency visits) reached 69% for asthmatic children [4].
Data suggests that uncontrolled asthma is frequent in Argentina and a need to identify its associated factors is highlighted. Nevertheless, the existence of a unique severe asthma phenotype in children has been questioned, taking into account that pediatric asthma is often linked to a highly frequent exacerbations rate in spite of a relatively normal lung function. Both severity and control are related in asthmatic patients, but both variables are not interchangeable. While asthma control refers to the extent to which asthma manifestations have been reduced or removed by treatment, asthma severity is based on the treatment intensity required to achieve a good disease control [5].
According to the WHO, severe asthma has been defined as “uncontrolled asthma which can result in risk of frequent severe exacerbations (or death) and/or adverse reactions to medications and/or chronic morbidity”. Therefore, severe asthma includes 3 distinct groups: 1) Untreated severe asthma due to diagnostic failure, lack of access to medical care, or non- adherence to therapy; 2) Severe Difficult to Control Asthma (SDCA) from comorbid conditions or adverse environmental circumstances, and 3) Severe Therapy Resistant Asthma (STRA), which includes patients whose control is not achieved despite intensive therapy and asthma for which control can only be maintained with intensive therapy [6].
Several factors have been related to education in asthma and may influence in adherence to treatment and control (patients' disease knowledge, cultural and socioeconomic aspects, poor perception of asthma symptoms, adverse events, and skills in use inhaler devices). Asthma education is not only a patient right, but also an effective strategy to reach short and longterm asthma control. It has been demonstrated that a multidisciplinary, comprehensive care of high risk children with chronic illnesses (including asthma) was associated with a reduction of emergency wards visits, hospitalization length of stay and healthcare costs, when compared with usual care. In the present study, we present the implementation of an interdisciplinary program for severe asthma patients in a large pediatric hospital to verify its impact in main outcomes [7-9].
Materials and Methods
Patient characteristics
A prospective, observational, longitudinal, case-series study was performed in the pneumonology service of the hospital pediátrico de Cordoba (Argentina) from July 2019 to February 2020 [10].
Inclusion criteria:
1) Age between 5 and 15 year-old.
2) Diagnosis of UCSA: Asthma that is uncontrolled despite GINA Step 4 or 5 treatment (medium of high dose Inhaled Corticosteroids (ICS) with a second controller, or maintenance Oral Corticosteroids (OCS)), with poor symptom control Asthma Control Test (ACT) score <19), or at least 2 exacerbation in the previous 12 months requiring OCS and/or emergency ward visit, or hospitalization.
3) Written informed consent.
Exclusion criteria:
1) Lack of informed consent for participation.
2) Unable to answer and complete control questionnaires.
3) Having discarded cystic fibrosis, obliterative bronchiolitis, bronchopulmonary dysplasia or chronic post-viral pulmonary disease and others diseases that mimic severe asthma.
Program description
Patients were included in the program when they meet the criteria mentioned in the paragraph above and were evaluated during 6-month period in our specialized center of asthma to define asthma severity. All participants were free and consistently supplied with controller medication (budesonide +formoterol (99%), fluticasone+vilanterol the distribution of the treatment is that they enter the program maximum dose of ICS for the age plus 1 or 2 more controllers). Exacerbation treatments included Short Acting Beta Agonists (SABAs) and OCS (meprednisone 8 mg or betamethasone 1 mg/kg/day); in patients older than 12 treated with budesonide+formoterol controller, the Maintenance and Reliever Therapy (MART) with aerochambers was considered the first-line exacerbation treatment (for patients on ICS/formoterol controller therapy).
Participants were controlled in monthly scheduled visits by a multidisciplinary team. In every visit on a same day, each patient was controlled by the mental health service (both parents and patient), the respiratory therapist service and the pulmonology service [11].
The team evaluated during each visit:
• The number of exacerbations.
• Use of reliever medication.
• Adherence to treatment: one of the purposes of the 3 services was to educate and raise awareness of the importance of taking the medication every day. A month supply of medication was given to each patient, instructing them to return when ICS canister or montelukast blisters were empty.
• Control of inhalation technique.
• Evaluations of comorbidities of diseases associated with asthma; if necessary, patients were referred for consultation with otorhinolaryngology, gastroenterology, obesity and cardiology.
After dispensing the medication to last for a month, a new visit was scheduled each month with a prior phone call. At each visit for 6 continuous months, we were guided by the GINA protocol for severe asthma to identify patients with SDCA and STRA, and in addition to performing the ACT questionnaire (asthma control was defined with a score ≥ 20). Spirometries were performed on the first and last visit, according to the global lung function initiative criteria with a spirodoc® device.
Also at the first and 6 months visits, asthma related quality of life was assessed using the Pediatric Asthma Quality of Life Questionnaire (PAQLQ) [12].
In the visit 2, blood sample were obtained for eosinophile count and Immunoglobulin E (IgE) quantification. Skin allergy tests were also performed (positive control, negative control, cat epithelium, dog epithelium, alternaria, aspergillus, aerogen fungi, mite allergen mixture, grass allergen mixture, tree allergen mixture, diater laboratories®) [13].
On visits 1 and 6, height and weight were evaluated and each patient was classified according to percentiles tables. Body Mass Index (BMI) was calculated, and participants were classified considering gender and age specific recommended by the world health organization for children and adolescents. In patients with suspected lung parenchyma lesions, a pulmonary Computed Tomography (CT) scan was requested [14].
All visits included an interview with psychologists, psychiatrists and educational psychologists. Psychological interviews were directed to the patients, their parents and eventually other relatives, including ludic and projective tools (free drawing, Machover test). Besides, treatment adherence, inhaling technique and physical activity habits were checked by physical therapists in all visits. Withdrawal from the program was considered when a patient voluntarily resigned their participation or when a participant did not attend two or more visits or did not comply with the treatment, which are indicators of a poorly adherent patient [15].
Ethic issues
All participants were volunteers who provided written consent and were aware that they are free to terminate participation at any stage of the study. The researcher read the informed consent form for participants who had problems with reading. The teaching and research committee of the pediatric hospital of Cordoba approved the research study. The study was performed in accordance with the standards as outlined in the declaration of Helsinki. (Registration ID: Severe Asthma Program 07-05-2019) [16].
Statistical analysis
Numerical variables were described according to central tendency and dispersion and comparisons were performed using student or Wilcoxon tests. Categorical variables were described according to their absolute and relative frequency and comparisons were performed using chi squared test. Follow-up was evaluated by MacNemar test. A p<0.05 was considered significant. All tests were carried out with SPSS®26.0 [17].
Objectives
Our main objective was to identify, through an interdisciplinary team, differential characteristics of SDCA and STRA patients and to evaluate the evolution of severe asthma during 6 months of follow-up. Secondary objectives were reducing the rate of hospitalizations and visits to the emergency room as well as exacerbations. The evaluation of the impact of three education related variables (treatment adherence; emotional triggering factors; adherence and correct use of devices and aero chambers) was defined as an exploratory objective [18].
Results
General characteristics
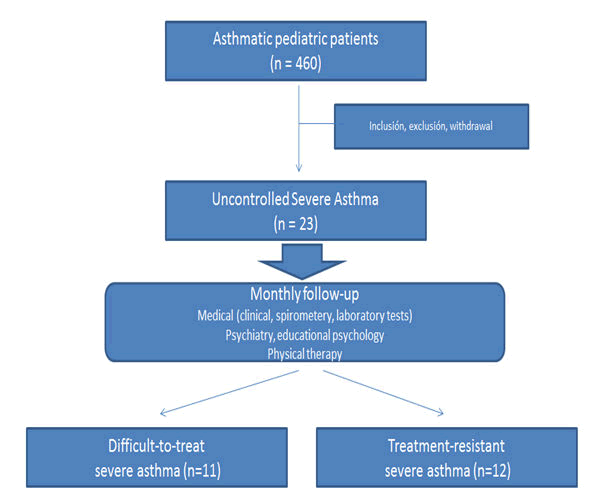
During the study period, 460 pediatric asthmatic patients were evaluated in the pneumology service. Twenty seven cases (5.88%) were included in the program according to inclusion/ exclusion criteria. After excluding 4 patients because of two or more lost visits, the remaining 23 subjects assisted to all visits (n=18) or did not assist to only one visit (n=5). Ten families (43.5%) had a very low monthly income (<10,000 ARS) and 56.6% had no health insurance. Demographic, clinical and specific characteristics of the whole cohort are summarized in Table 1. SDCA was confirmed in 11 patients and STRA in the other 12 participants (Figure 1) [19].
| Clinical, spirometry and laboratory data | |
| Age (years), mean (SD) | 9.3 (9) |
| Female gender (%)(n) | 52.2% (15) |
| Specialty consultations (%) | 70% |
| Initial ACT score ≥ 20 (%) | 30% |
| PAQLQ score, mean (SD) | 4.85 (1.38) |
| Near fatal asthma (%) (*) | 43.57% |
| Exacerbations triggered by emotional factors (%) | 57% |
| Obesity (%) | 30.70% |
| Allergic rhinitis (%) | 56% |
| Initial IgE (U/L), median (range) | 780.39 (23–3050) |
| Positive skin tests%, mean (SD) | 82.7 (17.3) |
| Eosinophil count (cells/µL), median (range) | 457 (0–1365) |
| Forced Vital Capacity (FVC) (%), mean (SD) | 90.74 (3.68) |
| Forced Expiratory Volume (FEV1), mean (SD) | 92.43 (3.88) |
| Post-β2 FEV1 increase, mean (SD) | 9.09 (2.84) |
| VEF1/FVC, mean (SD) | 100.35 (1.73) |
| Maximal mid-expiratory flow, mean (SD) | 102.78 (7.76) |
| Normal high resolution computed tomography (%) (**) | 80% |
Note: (*) Defined as acute asthma associated with a partial pressure of carbon dioxide (PaCO2)>50 mmHg, requiring mechanical ventilation. (**) Computed tomography was performed in 10 patients.
Abbreviation: PAQLQ: Asthma Quality of Life Questionnaire; SD: Standard Deviation. VEF1: Forced Expiratory Volume in the 1st second; FEV1: Forced Expiratory Volume; FVC: Forced Vital Capacity.
Table 1. Clinical, spirometry and laboratory data.
Impact on therapy
Program implementation was associated with a 25%-50% dose reduction of ICS and montelukast therapy in all UCSA patients. After 6 months of follow-up, 3 of 23 participants (10.34%) were re-categorized as moderate persistent asthma cases [20].
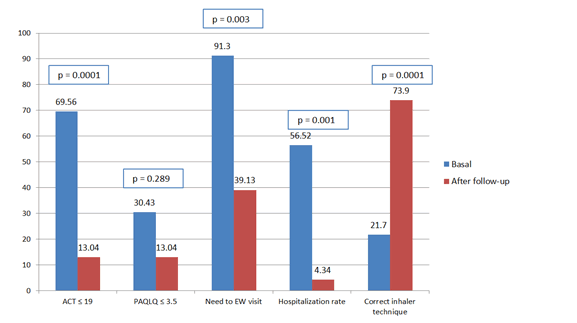
Before program starting, the rate of participants with an adequate inhaler technique was 21.7% (5 cases). After 6 months of follow-up, 73.9% (n=17) of our patients could adequately use their treatment inhalers (p=0.0001) (Figure 2).
Impact on exacerbation rates
Rate of exacerbations during the 12 months previous to the program implementation was 100%, with a mean of 11.72 events per patient. During follow-up, 17 subjects (68%) experienced at least one exacerbation [21].
Impact on healthcare resources
Program implementation was associated with a significant reduction of hospitalization rates. Before starting 41.37% (n=12) of all patients needed at least one hospitalization in general ward or intensive care unit; during follow-up, only one subject (4%) had to be assisted as an inpatient (p=0.001) [22].
In our cohort 91.3% of patients needed at least one visit to the emergency ward in the 12 months previous to program implementation. During the period of follow-up, the rate of visits was reduced to 39.13% (p=0.003). No patient was hospitalized in the intensive care unit or needed mechanical ventilation during the study period [23].
Impact on symptoms and quality of life
Before program starting, 16 patients (69.56%) presented an ACT score <20 points and 8 subjects (30.43%) were characterized by a PAQLQ score <3.5 points. After 6 months of follow-up, proportion of participants with an ACT score lower than 20 points was significantly reduced to 13% (p=0.0001) and the rate of patients with a PAQLQ score <3.5 points was 13.4% (p=0.289) [24].
Impact of psychological variables
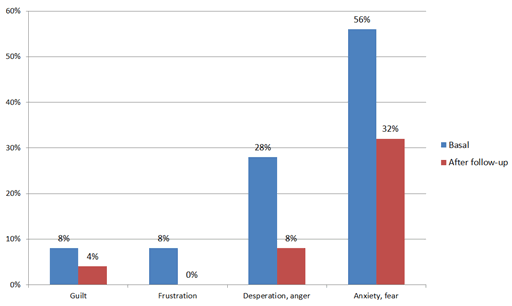
Caregivers’ reactions during exacerbations improved after program implementation (Figure 3). Most caregivers pointed out climatic factors (64%) and other infectious or allergic variables (28%) as the main triggers of pediatric asthma exacerbations, while potential emotional factors (sadness, anxiety, and irritability) were considered less frequently (8%) [25].
Subgroup comparisons
Eleven patients (47.82%) were defined as SDCA and 12 participants (52.17%) were considered as STRA. Biologic treatment was used in 4 STRA subjects (omalizumab) and other 4 participants had been considered for this strategy (omalizumab (n=3), mepolizumab (n=1)).
Characteristics of both subgroups are summarized in Table 2. Patients with STRA had a significantly higher probability of low socioeconomic status (p=0.005), using of rescue medication (p=0.003) and need for evaluation in an emergency ward (p=0.005) during the 6 months follow-up [26].
| Variable | SDCA | STRA | p-value |
|---|---|---|---|
| N (%) | 11 (47.82%) | 12 (52.18%) | |
| Age (years), mean (SD) | 9.45 (3.07) | 9.17 (2.29) | 0.8 |
| Male gender | 5 (45.45%) | 6 (50%) | 0.83 |
| Low caregiver educational status (*) | 7 (63.63%) | 8 (66.67%) | 0.79 |
| No healthcare insurance | 6 (54.54%) | 7 (58.33%) | 0.85 |
| Low physical activity (**) | 7 (63.63%) | 8 (66.67%) | 0.88 |
| Low familiar month income (†) | 1 (9.09%) | 8 (66.67%) | 0.005 (‡) |
| Exacerbation during the last 12 months (≥ 5/year) | 7 (63.63%) | 9 (75%) | 0.55 |
| Severe exacerbations during the last 12 months (≥ 2/year) | 3 (27.28%) | 4 (33.33%) | 0.75 |
| EW visits during the last 12 months (≥ 5/year) | 5 (45.45%) | 6 (50%) | 0.83 |
| Hospitalization in GW during the last 12 months (≥ 1/year) | 4 (36.36%) | 5 (41.67%) | 0.79 |
| Hospitalization in ICU during the last 12 months (≥ 1/year) | 3 (27.28%) | 3 (25%) | 0.9 |
| Systemic corticosteroids use during the last 12 months (≥ 5/year) | 7 (63.63%) | 7 (58.33%) | 0.9 |
| Near fatal asthma (≥ 1/year) | 6 (54.54%) | 4 (33.33%) | 0.3 |
| Basal ACT score ≤ 19 | 6 (54.54%) | 10 (83.33%) | 0.13 |
| Basal PAQLQ score ≤ 3.4 | 3 (27.28%) | 4 (33.33%) | 0.75 |
| Adherence to visit schedule | 10 (90.91%) | 11 (91.67%) | 0.95 |
| Hospitalization during follow-up | 0 (0%) | 1 (8.33%) | 0.33 |
| EW visits during follow-up | 1 (9.09%) | 8 (66.67%) | 0.005 (‡) |
| Clinical improvement during follow-up | 10 (90.91%) | 10 (83.33%) | 0.59 |
| Rescue medication use during follow-up | 5 (45.45%) | 8 (66.67%) | 0.003 (‡) |
| Allergic rhinitis | 5 (45.45%) | 8 (66.67%) | 0.3 |
| Obesity | 3 (27.28%) | 4 (33.33%) | 0.75 |
| IgE (U/L), mean (SD) | 481 (589) | 1052 (968) | 0.11 |
| Eosinophil count (cells/µL), mean (SD) | 601.27 (397) | 324.83 (273) | 0.06 |
| Positive skin test | 3 (27.28%) | 3 (25%) | 0.59 |
| FVC ≤ 79 | 2 (18.18%) | 3 (25%) | 0.69 |
| FEV1 ≤ 79 | 3 (27.28%) | 3 (25%) | 0.69 |
| MMEF | 2 (18.18%) | 2 (16.67%) | 0.92 |
Note: All values are n (%), except when specified. (*) Incomplete elementary and/or preparatory school; (**) No more than twice a week; (†) Less than 10,000 ARS; (‡) Significant difference.
Abbreviation: SDCA: Difficult-To-Treat Severe Asthma; STRA: Treatment-Resistant Severe Asthma; EW: Emergency Ward; FVC: Forced Vital Capacity; GW: General Ward; ICU: Intensive Care Unit; MMEF: Maximal Mid-Expiratory Flow; PAQLQ: Asthma Quality of Life Questionnaire; SD: Standard Deviation; FEV1: Forced Expiratory Volume.
Table 2. Characterization of patients’ subgroups.
Discussion
Our main objective was to describe clinical, functional and treatment characteristics of UCSA pediatric patients. Proportion of SA was 5.88% of the whole pediatric asthmatic population assisted in our large reference hospital. A low socioeconomic and educational level of caregivers and the lack of healthcare insurance were frequent among our severe asthmatic patients. As previously demonstrated, a low socioeconomic status is a risk factor for respiratory symptoms. These data are relevant when evaluating the characteristics associated with the severity of asthma, since severe forms of this disease can compromise up to 29% of all family income, as reported in other Latin American cohorts [25].
Basal levels of IgE, eosinophil count and skin test positivity were not useful variables to differentiate SDCA of STRA, contrasting with previous research. Both more frequent use of rescue medication and a higher rate of visit to emergency wards were also differential characteristics of STRA. More than 52% of our patients were characterized as STRA, a high rate when compared with previous published studies. It is worth noting that program intervention on modifiable factors led to a re-categorization of several SDCA patients as moderate persistent asthma, with reduction of inhaled corticoids and long-acting beta-2 treatment doses, and a reduction of prescriptions of montelukast. OCS and biological treatment were only needed in STRA patients. These results are consistent with previous local research [26].
We found 30% obesity close to similar to the percentages in Argentinian study that was 39%. This data is important, since obesity can influence the severity and resistance to treatment; nevertheless, obesity was not a useful variable to differentiate between SDCA and STRA. Our program specially emphasized on compliance, inhaler technique and treatment accessibility at each visit. In all visits, necessary medication was freely supplied and a triple educational intervention was carried out by physicians, physical therapists and psychology educators. We observed an improvement in the correct use of inhalers in more than 52% of our patients, with a probable impact on improving our evaluated variables, as it has been previously reported [27].
The reduction of exacerbations and hospitalizations rates is one of the main objectives of asthma control strategies. After our program implementation, rate of hospitalizations was significantly reduced. Previous Latin American experience was associated with similar results. A Brazilian program with similar characteristics induced a reduction of hospitalization rate by 42%. In a reference hospital of Buenos Aires, the implementation of a similar program was associated with significantly reduced rates of emergency ward visits and hospitalizations. In our cohort, both rates were reduced by more than 52% after 6 months of program intervention [28].
It is also highlighted that the program was associated with a significantly improvement in symptom control, measured through the ACT score. After 6 months of follow-up, proportion of patients with an ACT score ≤ 19 point decreased by more than 52%. Even though PAQLQ scores improved in our cohort in a non-significant level, our results are consistent with the meta-analyses of Boyd et al. (n=7843) [29], that demonstrated that asthma education aimed at children and their caregivers may result in lower risk of future emergency department presentation and hospital admission, but its effects on quality of life remain uncertain.
When investigating exacerbation triggers, we identified a change of more frequent causes during follow-up. While climatic and allergenic factors were considered main triggers in basal evaluations, emotional variables were the most reported cause of exacerbations at end of follow-up. In previous studies, higher parenting stress in mothers was also associated with higher airway inflammation and more frequent behavioral disorders in asthmatic children [30].
We admit some limitations of our study. First, our sample was small; however, it is the expected percentage of SA in our hospital population (5%-10%) and, thanks to the encouraging results of this interdisciplinary program, we may expect that this strategy may be carried out throughout the whole province. Secondly, adherence is extremely difficult to assess, as shown in another study by Engelkes. Third, we did not have devices with electronic counters. Fourth, “Test of the adherence to inhalers” questionnaires were not used; nevertheless, such tools are approved only in people aged over 18 years old [31].
Conclusion
The implementation of our program in a large public pediatric hospital led to differentiate and characterize SDCA and STRA subgroups. Interdisciplinary management allowed us to meet the exploratory objectives such as correct inhaler technique, adherence to treatment, emotional variables and especially patient education. Implementing an interdisciplinary program is associated with a marked improvement in all modifiable variables of asthma in patients with UCSA. Patients with STRA were adequately phenotyped and, if symptoms persisted after follow-up, they were indicated a biological treatment. The favorable results of our initial small sample would be very useful to start a new way to address the management of SA in patients in our province. This modality has been included as a special program of attention in our hospital.
Acknowledgement
Editorial support, under the direction of the authors, was provided by Agencia Médica, funded by AstraZeneca.
References
- Comite Nacional de Neumonologia, Comite Nacional de Alergia, Comite Nacional de Medicina Interna, et al. Guia de diagnostico y tratamiento: Asma bronquial en ninos ≥ 6 anos. Actualizacion 2016. Resumen ejecutivo. Arch Argent Pediatr 2016; 114(6): 595-6.
- Mallol J, Sole D, Baeza-Bacab M, et al. Regional variation in asthma symptom prevalence in Latin American children. J Asthma 2010; 47(6): 644-50.
- Giubergia V, Fridman N, Gonzalez Pena H. Evaluation of the impact of a care program for children with severe asthma. Arch Argent Pediatr 2012; 110(5): 382-7.
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2016.
- BTS/SIGN British guideline on the management of asthma. 2019.
- Neffen H, Fritscher C, Schacht FC, et al. Asthma control in Latin America: The asthma insights and reality in Latin America (AIRLA) survey. Rev Panam Salud Publica 2005; 17: 191-197.
- Guia de diagnostico y tratamiento: Asma bronquial en ninos ≥ 6 anos. Actualizacion 2016. Resumen ejecutivo.
- Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children: Mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med 2004; 170: 426–432.
- Reddel HK, Taylor DR, Bateman ED, et al. An official American thoracic society/european respiratory society statement: Asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009; 180: 59–99.
- Taylor DR, Bateman ED, Boulet LP, et al. A new perspective on concepts of asthma severity and control. Eur Respir J 2008; 32(3): 545–554.
- Fitzpatrick AM, Baena-Cagnani CE, Bacharier LB. Severe asthma in childhood: Recent advances in phenotyping and pathogenesis. Curr Opin Allergy Clin Immunol 2012; 12(2): 193-201.
- Souza-Machado A, Santos PM, Cruz AA. Adherence to treatment in severe asthma: Predicting factors in a program for asthma control in Brazil. World Allergy Organ J 2010; 3(3): 48-52.
- Stelmach R, Neto AC, Fonseca AC, et al. A workshop on asthma management programs and centers in Brazil: Reviewing and explaining concepts. J Bras Pneumol 2015; 41(1): 3-15.
- Mosquera RA, Avritscher EB, Samuels CL, et al. Effect of an enhanced medical home on serious illness and cost of care among high-risk children with chronic illness: A randomized clinical trial. JAMA 2014; 312(24): 2640-8.
- Crossref
- Juniper EF, Norman GR, Cox FM, et al. Comparison of the standard gamble, rating scale, AQLQ and SF-36 for measuring quality of life in asthma. Eur Respir J 2001; 18(1): 38-44.
- Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: A survey for assessing asthma control. J Allergy Clin Immunol 2004; 113(1): 59-65.
- Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur Respir J 2012; 40(6): 1324-43.
- de Onis M, Onyango AW, Borghi E, et al. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007; 85(9): 660–667.
- Machover K. Personality projection in the drawing of the human figure: A method of personality investigation. Charles C Thomas publisher. 1949.
- Pallasaho P, Lindström M, Polluste J, et al. Low socio-economic status is a risk factor for respiratory symptoms: A comparison between Finland, Sweden and Estonia. Int J Tuberc Lung Dis 2004; 8(11): 1292-300.
- de Andrade WC, Lasmar LM, Ricci Cde A, et al. Phenotypes of severe asthma among children and adolescents in Brazil: A prospective study. BMC Pulm Med 2015; 15: 36.
- Lombardi C, Savi E, Ridolo E, et al. Is allergic sensitization relevant in severe asthma? Which allergens may be culprit? World Allergy Organ J 2017; 10(1):2.
- Selby L, Saglani S. Severe asthma in children: Therapeutic considerations. Curr Opin Allergy Clin Immunol 2019; 19(2): 132-140.
- Neffen H, Vidaurreta S, Balanzat A, et al. Poorly controlled asthma: Diagnosis and therapeutics in children and adolescents. Medicina (B Aires) 2012; 72(5): 403-13.
- Elizathe LS, Arana FG, Rutsztein G. A cross-sectional model of eating disorders in Argentinean overweight and obese children. Eat Weight Disord 2018; 23(1): 125-132.
- Anderson WC 3rd, Szefler SJ. New and future strategies to improve asthma control in children. J Allergy Clin Immunol 2015; 136(4): 848-59.
- Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med 2004; 170: 426–432.
- Boyd M, Lasserson TJ, McKean MC, et al. Interventions for educating children who are at risk of asthma-related emergency department attendance. Cochrane Database Syst Rev 2009; 2009(2): CD001290.
- Verkleij M, van de Griendt EJ, Colland V, et al. Parenting stress related to behavioral problems and disease severity in children with problematic severe asthma. J Clin Psychol Med Settings 2015; 22(2-3): 179-93.
- Engelkes M, Janssens HM, de Jongste JC, et al. Medication adherence and the risk of severe asthma exacerbations: A systematic review. Eur Respir J 2015; 45(2): 396-407.