Current Pediatric Research
International Journal of Pediatrics
Efficacy of caudal neostigmine for postoperative pain relief: A systemic review and meta-analysis.
Semagn Mekonnen1 and Yigrem Ali2
1Department of Anesthesiology, College of Health and Medical Sciences, Dilla University, Ethiopia.
2Department of Psychiatry, College of Health and Medical Sciences, Dilla University, Ethiopia.
- *Corresponding Author:
- Yigrem Ali
Head Department of Psychiatry
Dilla University, Ethiopia.
Tel: +251913864605
E-mail: aliyigrem@gmail.com
Accepted date: May 25, 2017
Caudal Epidural analgesia reduces the overall intraoperative Anesthetic requirement and facilitates rapid return of conscious state with stable hemodynamic condition. Different additives like, opioids, ketamine and clonidine have shown better pain management but brought about evitable complications. Addition of neostigmine improves pain management with minor postoperative complications. The purpose of this review was to compare caudal epidural neostigmine co-administered with bupivacaine and Bupivacaine alone. Different databases were explored to identify controlled clinical trials comparing neostigmine co-administered with bupivacaine and bupivacaine alone. Seventeen controlled trails have been collected for eligibility assessment and eleven trials were incorporated for extraction of outcomes. Eleven studies (838 participants) were included in this review. The mean difference of Postoperative analgesic duration was better in patients received neostigmine co-administered with bupivacaine when compared to bupivacaine alone (MD=3.29, 95% confidence interval (CI) 2.86 to 4.4, 4 trials, 193participants). There was significant association on postoperative analgesic consumption in 24 h when neostigmine group was compared with bupivacaine alone (SMD=-1.57, 95% confidence interval (CI) -2.14, 1.01, one trial, 63 participants). Conclusion: Caudal epidural with neostigmine co-administered with bupivacaine is better as compared to caudal epidural with bupivacaine.
Keywords
Caudal epidural, Neostigmine, Bupivacaine, Postoperative analgesia.
Introduction
Pain is the most distressful consequence of surgery with preventable complications in perioperative period [1,2]. However, expression of pain by younger children and even the method of pain assessment is more complex which makes children vulnerable to postoperative pain [3]. Studies showed that aggressive postoperative pain treatment in children with opioids and other analgesic alone were associated with many adverse effects [1,4-9].
Caudal epidural analgesia reduces the overall intraoperative anesthetic requirement and facilitates rapid return of conscious state with stable hemodynamic condition [1,4- 7,9-15]. However, perioperative pain relief with single shot caudal block is inadequate which needs further administration of other analgesic drugs intravenously [7,9,13,14]. So far, different additives like, opioids, ketamine and clonidine have been used to enhance postoperative pain relief but associated with undesirable side effects [3-7,9,12-14].
Neostigmine provide pain relief by preventing the breakdown of acetylcholine in spinal cord mediated by muscarinic and cholinergic receptors with minimal postoperative side effects [4,5,8,14,16].
Some studies showed that administration of neostigmine and bupivacaine prolong postoperative analgesia in children having lower extremity surgeries [3-6,8-10,12,14], whereas, other study pointed out that neostigmine did not prolong the duration of postoperative analgesia [7,17].
Objective
To identify postoperative pain relief benefits of caudal neostigmine in children undergoing lower abdominal surgeries.
Studies in this Review
Study Design
Randomized controlled trials.
Population
All children scheduled for lower abdominal surgery.
Types of Intervention
Intervention: Children receiving caudal epidural Block with combined Neostigmine (2-4 μg/kg) and Bupivacaine (1 mg/kg).
Control: Children receiving caudal epidural Block with bupivacaine alone.
Outcomes
Main outcomes: The primary outcomes were analgesic duration and the total analgesic consumption in 24 h.
Secondary outcomes: The secondary outcomes were nausea and vomiting and change in hemodynamic parameters.
Criteria for Selection of Clinical Trials
Different databases were explored to identify controlled clinical trials comparing neostigmine co-administered with bupivacaine and bupivacaine alone. Full reports of all controlled clinical trials were searched without date and language restriction. Seventeen controlled trails have been collected for eligibility assessment and eleven trials were incorporated for extraction of outcomes.
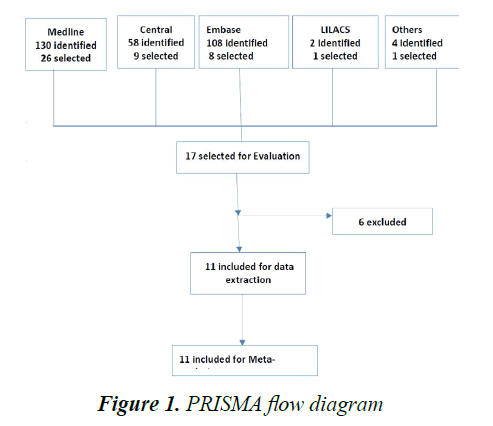
Two review authors independently assess the eligibility assessment with checklist and disagreement was fixed by consensus. The method of eligibility assessment by each independent author was mentioned in Table 1 and the excluded Studies were described with reasons in Table 2.The study selection process was summarized using PRISMA chart (Figure 1).
| Study | Year of Publication | Sample Size (N) | Intervention (neostigmine co-Administer With Bupivacaine | Control (bupivacaine) | Outcomes |
|---|---|---|---|---|---|
| Bhardwaj et al. [7] | 2007 | 120 | 2, 3 and 4 µg/kg of Neostigmine with 1.875 mg/kg of bupivacaine in three groups | 1.875 mg/kg of bupivacaine alone | Time to first analgesic, number of analgesic doses, sedation, vomiting |
| Dilek et al. [17] | 2003 | 40 | 1 µg/kg of Neostigmine with 0.25% 0f 0.5 mg/kg bupivacaine | 0.25% 0f 0.5 mg/kg bupivacaine alone | Duration of analgesia, pain score, nausea and vomiting |
| Emil et al. [10] | 2015 | 134 | 1.5, 3 and 6 µg/kg of Neostigmine with 0.25% of 0.5 mg/kg of bupivacaine in three groups | 0.25% of 0.5 mg/kg of bupivacaine alone | Duration of analgesia, analgesic consumption, pain score |
| Ataro et al. [8] | 2014 | 86 | 2.5 µg/kg of Neostigmine with 0.25% of 1 mg/kg bupivacaine | 0.25% of 1 mg/kg bupivacaine alone | Duration of analgesia, first rescue analgesic hors, vomiting, pain score |
| Kaushal et al. [6] | 2008 | 90 | 2 and 5 µg/kg of Neostigmine with 0.25% of 1 mg/kg bupivacaine | 0.25% of 1 mg/kg bupivacaine alone | Duration of analgesia, first rescue analgesic hour, vomiting, pain score |
| Abdulatif et al. [12] | 2002 | 60 | 2 µg/kg, 2 µg/kg diluted with NS and 0.25% of 1mg/kg bupivacaine in two groups | 0.25% of 1 mg/kg bupivacaine alone | Duration of analgesia, analgesic consumption, vomiting |
| Mrugesh et al. [1] | 2016 | 75 | 1 and 2 µg/kg of Neostigmine with 0.25% of 1 mg/kg bupivacaine | 0.25% of 1 mg/kg bupivacaine alone | Duration of analgesia, vomiting , number of rescue analgesics, pain scores |
| Tobin et al. [14] | 2014 | 63 | 1.5 µg/kg of Neostigmine with 0.25% of 1 mg/kg bupivacaine | 0.25% of 1 mg/kg bupivacaine alone | Time to first rescue analgesic, number of rescue analgesic, pain score, vomiting, fever |
| Rajesh et al. [3] | 2004 | 80 | 2, 3 and 4 µg/kg of Neostigmine with 0.25% of 0.5 mg/kg bupivacaine in three groups | 0.25% of 0.5 mg/kg bupivacaine alone | First rescue analgesics, analgesic consumption, vomiting |
| Anaesth et al. [9] | 2005 | 40 | 2 µg/kg of Neostigmine with 0.25% of 1 mg/kg bupivacaine | 0.25% of 1 mg/kg bupivacaine alone | Duration of analgesia, first analgesic hours nausea and vomiting |
| Sunanda et al. [4] | 2013 | 50 | 2 µg/kg of Neostigmine with 0.125% of 2 mg/kg bupivacaine | 0.125% of 2 mg/kg bupivacaine alone | Time to first rescue analgesic, number of rescue analgesic, pain score |
Table 1.Description of included studies
| Study | Year of Publication | Sample Size | Reasons for Exclusion |
|---|---|---|---|
| Gupta et al. [18] | 2011 | 50 | The intervention was bupivacaine. Used different doses of bupivacaine with fixed neostigmine dose |
| Kiran et al. [13] | 2006 | 50 | Different population group was used (adult). Neostigmine was compared with bupivacaine and lidocaine for epidural analgesia rather than bupivacaine alone |
| Sfyra et al. [5] | 2007 | 50 | Comparison was made with levobupivacaine |
| Sayed et al. [11] | 2015 | 80 | Randomization and blinding was done properly but the surgery was open heart surgery which was different from this population(children with lower extremity surgery) |
| Dilek et al. [17] | 2003 | 44 | Neostigmine was compared with ropivacaine, not bupivacaine |
| Kiran et al. [13] | 2016 | 120 | Neostigmine was compared with levobupivacaine, not bupivacaine |
Table 2. Description of excluded studies
Searching Strategy
Databases were searched for randomized clinical trials comparing neostigmine and bupivacaine without date and language restriction as shown below with medical subject heading (MeSH) terms of children, postoperative, pain, nausea and vomiting, caudal block were searched as follows:
Procedures of the Review
The principal author chose randomized clinical studies with full articles and no multiple publications. The two authors, SM and YA, check each study for eligibility as mentioned in methodology and the rest were excluded with reasons. The profile of included studies was assessed carefully with standard parameters as described in Table 3.
| Study | Sequence Generation | Allocation Concealment | Blinding | Incomplete Outcome Data | Selective Outcome Reporting | Free of Other Bias | Jadad Scale | ||
|---|---|---|---|---|---|---|---|---|---|
| Randomization | Blinding | Withdrawal | |||||||
| Bhardwaj et al. [7] | A | B | A | A | A | A | 2 | 2 | 0 |
| Emil et al. [10] | B | B | A | A | A | A | 2 | 1 | 0 |
| Ataro  et al. [8] | B | D | B | A | A | B | 1 | 1 | 0 |
| Kaushal et al. [6] | A | A | A | A | A | A | 2 | 2 | 0 |
| Emil et al. [10] | A | A | A | A | A | A | 2 | 2 | 0 |
| Mrugesh et al. [1] | B | B | D | A | A | A | 1 | 1 | 0 |
| Tobin et al. [14] | A | A | A | A | A | A | 2 | 2 | 1 |
| Rajesh et al. [3] | A | A | A | A | A | A | 2 | 2 | 0 |
| Anaesth et al. [9] | A | A | D | A | A | A | 2 | 1 | 0 |
| Sunanda et al. [4] | A | A | A | A | A | A | 2 | 2 | 0 |
| Dilek et al. [17] | A | A | A | A | A | A | 2 | 2 | 5 |
Table 3. Risk of base table of included studies
Statistical combination of data from two or more separate trials in a meta-analysis was decided based on the evaluation of the clinical and methodological heterogeneity. The inconsistency throughout trials was quantified with the I2 statistic, assuming a value more than 50% as a substantial heterogeneity.
The summary effect measure were risk ratio (RR) and odd ratio for dichotomous variables and mean Difference and standard deviation for continuous variables along with their corresponding 95% confidence intervals (CI). The meta-analysis was planned to be performed through a random-effects model and Mantel-Haenszel (M-H) statistical method, anticipating that trials would have different techniques and assuming that the actual true effects have a normal distribution.
The data Analysis was conducted using Review Manager (RevMan5.3, Cochrane Collaboration) and Comprehensive Meta-Analysis (CMA).This systematic review was carried out using the methods established by the Cochrane Handbook for Systematic Reviews of Interventions and we followed the recommendations and checklist items from the PRISMA Statement for Reporting Systematic Reviews and Meta-analysis.
Description of Studies
Three hundred and two trials were identified by searching strategy as shown in Figure 1. Seventeen studies were selected for evaluation with successive screening for eligibility. Finally, 11 studies with 838 participants were included for analysis and the rest were excluded with reasons (Table 2).
Eleven randomized clinical trials were published between 2002-2016. The age of included studies ranged from 1-15 years. Sample size varied from 40-134. General Anesthesia was given with nitrous oxide, oxygen and halothane in three studies [3,12,14]. In One study, general anesthesia was given with halothane with sleeping dose of thiopentone with laryngeal mask airway. Two studies combine fentanyl with propofol and halothane as induction agent [7,17]. The use of thiopental and succinylcholine for induction of anesthesia and intubation of the trachea was described in two studies. Two studies did not describe the method of induction [8,9]. Withdrawal of cases from the study were reported in two studies (Table 1) [14,17].
Results
Main Outcomes
As mentioned in methodology, postoperative analgesic duration and analgesic consumption were the primary outcomes. Four studies described mean postoperative analgesic duration and analgesic consumption with neostigmine compared with bupivacaine in two groups [4,9,14,17]. However, the five studies tried to identify the analgesic profile of neostigmine with different doses of neostigmine in 3-4 groups with a fixed bupivacaine dose. The ten studies presented the analgesic profile results in mean ± SD whereas only one study presented the analgesic profile results in median and range [8]. Ten studies concluded that caudal neostigmine co-administered with bupivacaine enhances the postoperative pain relief and also reduce requirement of rescue analgesic doses. In two studies, the postoperative analgesic profiles were similar between the groups and concluded that caudal neostigmine co-administered with bupivacaine didn’t prolong postoperative analgesia [7,17].
Secondary Outcomes
Postoperative nausea and vomiting: Incidence of postoperative nausea and vomiting were studied in ten studies. From included studies, nine studies found out that groups received neostigmine had experience nausea and vomiting when compared to groups received bupivacaine alone [3,5-9,12,14,17]. One study showed no difference between the groups. Only one study did not study incidence of nausea and vomiting [10].
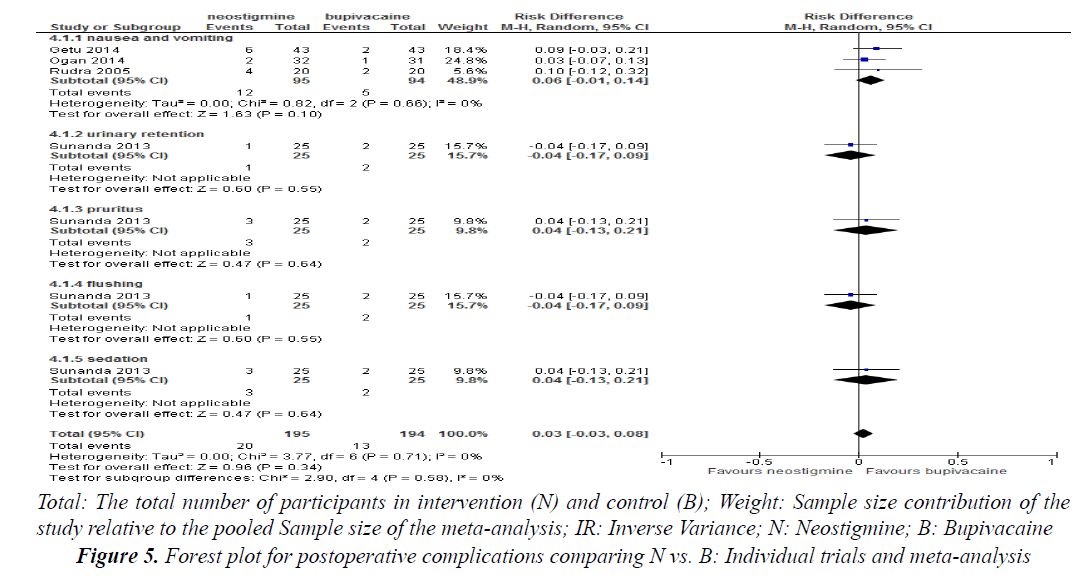
Sedation: Sedation was assessed with objective score based on eye opening in seven studies [3,5-7,9,12,17]. Six studies did not show any significant difference between the groups on sedation score [3,5,6,9,12,17]. Only one study found significant sedation score in patients received neostigmine [7]. Four studies didn’t study sedation as an outcome [4,8,10,14,18].
Urinary retention: Urinary retention was reported in one study. There were two cases of urinary retention in patients who received bupivacaine alone as compared to neostigmine co-administered with bupivacaine which was only one [4]. The incidence of urinary retention was (5%) in neostigmine co-administered with bupivacaine compared with bupivacaine alone which was (2.5%).
Pruritis and flushing: Pruritis and flushing were reported in one study where there were three pruritus cases in neostigmine group as compared to bupivacaine alone (two cases). But flushing was higher in bupivacaine group as compared to neostigmine group, 5% and 7.5%, respectively [4].
Respiratory depression and hypotension: Respiratory depression and unstable hemodynamic condition were not reported in included studies.
Quantitative Data Synthesis
Neostigmine co-administered with bupivacaine and bupivacaine alone were compared as intervention and controlled respectively with postoperative analgesic profile as primary outcomes in children having caudal epidural block for lower extremity surgery.
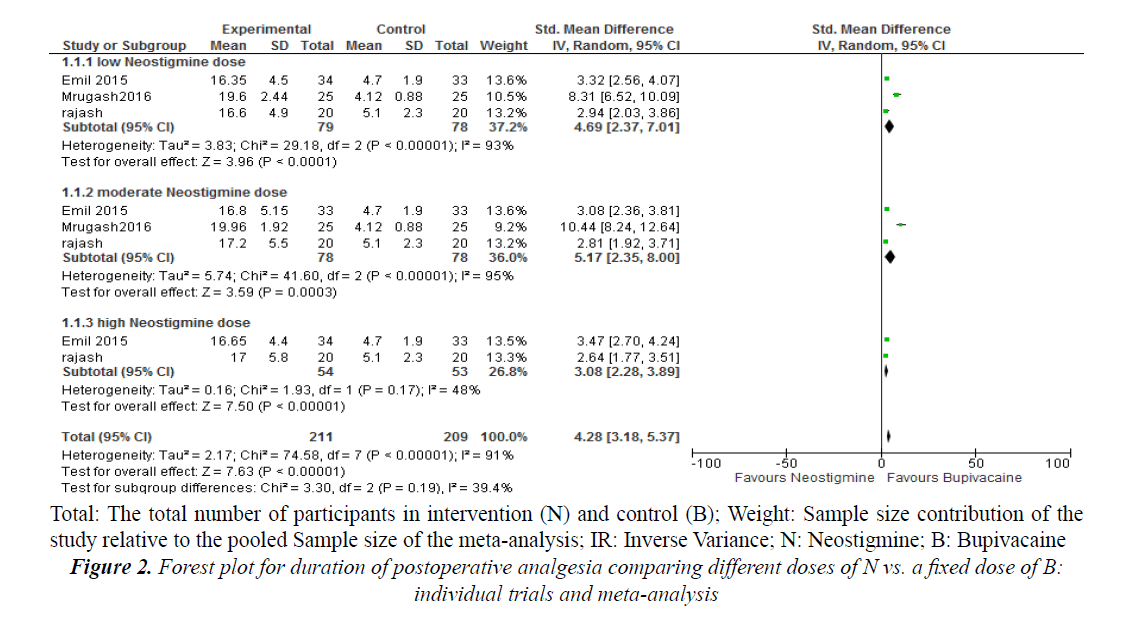
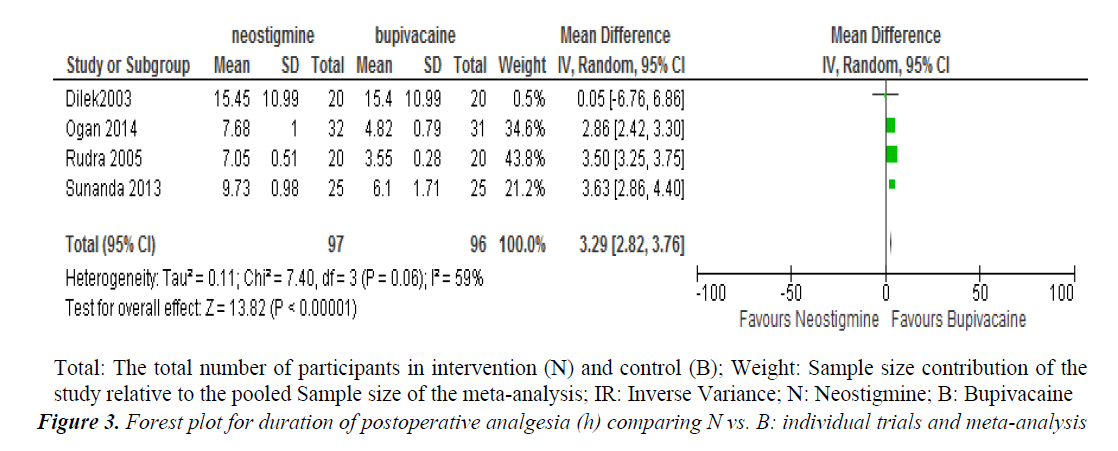
Subgroup analysis of different groups with different doses of neostigmine with a fixed dose of bupivacaine showed that the standardized mean difference of postoperative analgesic duration was better in neostigmine group when compared to bupivacaine alone (SMD=4.28, 95% confidence interval (CI) 3.18, 5.37, three trials, 157 participants) (Figure 2). The postoperative analgesic duration was better in patients received Neostigmine co-administered with bupivacaine when compared to bupivacaine alone (MD=3.29; 95% confidence interval (CI) 2.82 to 3.76; 4 trials; 193 participants) (Figure 3).
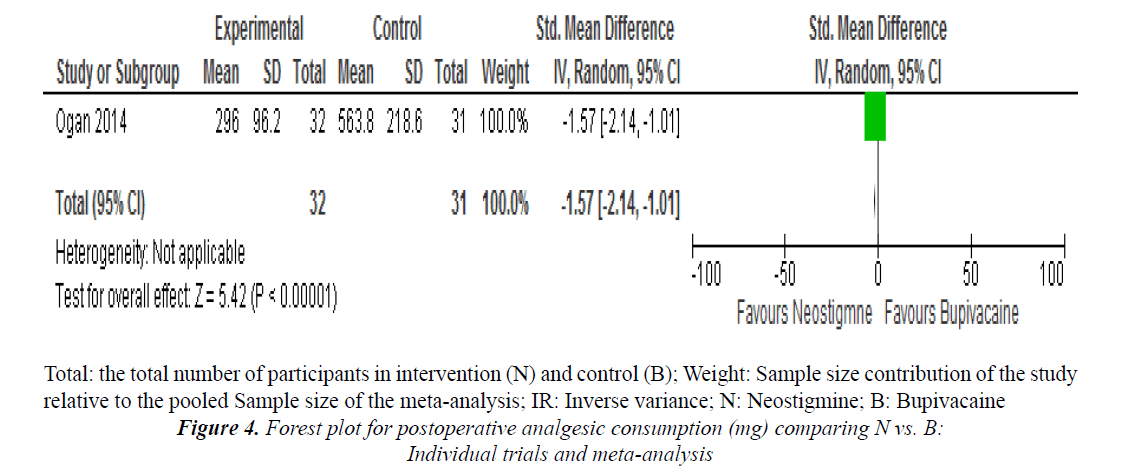
One study reported on postoperative analgesic consumption in 24 h and noted that there was significant association when neostigmine group was compared with bupivacaine alone (SMD=-1.57, 95% confidence interval (CI) -2.14, 1.01, one trial, 63 participants (Figure 4).
Post-operative nausea and vomiting was reported in three studies and there was no significant risk difference when neostigmine is compared with neostigmine alone (RD=0.06, 95% confidence interval (CI) -0.01, 0.14, 3 trials, 189 participants) (Figure 5).
Urinary retention, pruritus, sedation and flushing were reported in one study and did not show any significant risk difference when neostigmine was compared with bupivacaine alone (p>0.05) (Figure 5).
Discussion
This systemic review and meta-analysis tried to identify the benefit of caudal neostigmine administered with bupivacaine for postoperative pain relief in children undergoing lower extremity surgery.
Mortality was not reported in any of the included trials and this may reveal the relative safety of caudal epidural neostigmine with bupivacaine for perioperative pain management.
The review has shown that postoperative analgesic duration is better in patients receiving neostigmine along with bupivacaine compared to bupivacaine alone which is consistent with the majority of included randomized trials. However, two individual randomized trials did not show any significant difference on postoperative analgesic duration and this discrepancy might be due to small sample size or data collection bias.
Postoperative nausea and vomiting was higher in neostigmine co-administered with bupivacaine as compared to bupivacaine alone in individual trials. Nevertheless, the systemic review did not show any significant difference. This might be small sample size and low power which needs further review with large sample size.
The review also showed that requirement of rescue analgesia was less in children receiving caudal neostigmine co-administered with bupivacaine as compared to bupivacaine alone.
Despite insignificant difference, urinary retention was seen to be higher in cases received bupivacaine alone when compared to neostigmine co-administered with bupivacaine. This is because neostigmine has the advantage of contracting the detrusor muscles of bladder and voiding could easily be occurred unlike bupivacaine alone which relaxes the muscles of bladder helping for urination.
Sedation and pruritus were seen more often in cases received neostigmine co-administered with Bupivacaine as compared to bupivacaine alone, but the risk difference was not significant.
Conclusion
Neostigmine co-administered with bupivacaine in caudal epidural enhances postoperative pain relief compared to bupivacaine alone. The postoperative rescue analgesic requirement is also lower in patients receiving neostigmine with bupivacaine compared to bupivacaine alone.
Declaration
Ethical approval and consent to participate the research has been approved by Dilla University institutional Board Review.
Availability of Data and Material
The Authors wish not share the data as per research and dissemination office.
Authors’ Contribution
Semagn Mekonnen perceived and designed the study. Data extraction, analysis, interpretation of data and drafting the manuscript was accomplished by the two authors.
Source of Funding
This work was done by authors own resources.
References
- Mrugesh P, Shah TD, Chadha I, et al. Caudal neostigmine with bupivacaine for postoperative analgesia in pediatric patient comparison with bupivacaine alone. IJBAMR. 2016; 5: 239-247.
- Warren M, Mark F, David L, et al. Anesthesiology and Critical Care 2008; 485.
- Rajesh M, Vinod K, Grover, et al. Caudal neostigmine with bupivacaine produces a dose-independent analgesic effect in children. Can J Anesth 2004; 53: 702-706.
- Sunanda M, Sampa D, Kanak K, et al. Comparison of post-operative analgesic efficacy of caudal epidural bupivacaine versus bupivacaine and neostigmine combination in infraumbilical surgeries in children. Saudi Publica Brasil 2013: 192-198.
- Sfyra E, Georgiou TH, Svirkos M, et al. Caudal administration of levobupivacaine and neostigmine for postoperative analgesia in children. The Greek E-Journal of Perioperative Medicine 2007; 5:44-49.
- Kaushal D, Singh V, Abbas H, et al. Caudal bupivacaine-neostigmine for perioperative analgesia in pediatric patients undergoing infraumbilical surgeries. A prospective, randomized, double blind, controlled study. Int J Anesthesiol 2008; 21: 1-7.
- Bhardwaj N, Yaddanapudi S, Ghai B, et al. Analgesia produced by caudal bupivacaine in children. J Postgrad Med 2007; 9: 44-49.
- Ataro G, Bernard M. Anesthesia and clinical effectiveness of caudal epidural block using bupivacaine with neostgmine for pediatric lower extremity orthopedic surgery in cure. J Clin Anesthesia Res 2014; 5: 10-13.
- Anaesth IJ, Rudra A, Ghosh MK. Scope of caudal neostigmine with bupivacaine for post-operative analgesia in children . Comparison with bupivacaine. Indian J Anesth 2005; 49: 191-194.
- Emil B, Mohammad D, Zahi M, et al. Caudal bupivacaine-neostigmine effect on post-operative pain relief in Children. J R Med Serv 2015; 22.
- Sayed AEK, Ali M, Mohamed H. Caudal neostigmine and bupivacaine facilitates early extubation and provides prolonged post-operative analgesia in children undergoing open heart surgery. J Anesth Clin Res 2015; 6: 512.
- Abdulatif M, El-sanabary M. Caudal neostigmine, bupivacaine and their combination for post-operative pain management after hypospadias surgery in children. Anesth Analg 2002; 95: 1215-1218.
- Kiran MD, Sanikop CS, Kotur PF. Randomized control trial of neostigmine and Placebo as adjuvants to compound mixture of lignocaine and bupivicaine for epidural analgesia in lower abdominal surgeries. Indian J Anaesth 2006; 50: 448-451.
- Tobin M, Ogan ST, Mato CN. A comparative study between caudal bupivacaine and bupivacaine co-administered with neostigmine for postoperative analgesia in children. Niger Postgrad Med J 2014; 21: 1-6.
- Wong S, Li J, Chen C, et al. Caudal epidural block for minor gynecologic procedures. Chang Gung Med J 2004; 27: 116-121.
- Khangure N. Adjuvant agents in neuraxial blockade. Anaesthesia Tutorial of the Week. 2011; 230:1-10.
- Dilek M, Alparslan T, Beyhan K, et al. Caudal neostigmine for postoperative analgesia in paediatric surgery. Pediatr Anesth 2003: 324-328.
- Gupta R, Verma R, Bogra J. A Comparative study of intrathecal dexmedetomidine and fentanyl as adjuvants to bupivacaine. J Anaesthesiol Clin Pharmacol 2011; 27: 339-343.