Current Pediatric Research
International Journal of Pediatrics
Efficacy and safety of hydroxyurea in children with E-beta thalassemia.
Subham Bhattacharya*, Habib Rahaman, Sumana Datta
Department of Pediatric Medicine, IPDMER and SSKM Hospital, Kolkata, India
- Corresponding Author:
- Subham Bhattacharya
Department of Pediatric Medicine
IPDMER and SSKM Hospital
Kolkata, India
E-mail: drsubham@rediffmail.com
Received: 01 November, 2022, Manuscript No. AAJCP-22-78837; Editor assigned: 03 November, 2022, Pre QC No. AAJCP-22-78837(PQ); Reviewed: 11 November, 2022, QC No. AAJCP-22-78837; Revised: 21 November, 2022, Manuscript No. AAJCP-22-78837(R); Published: 30 November, 2022, DOI:10.35841/0971-9032.26.11.1688-1691.
Background: E-beta thalassemia is one of the commonest thalassemia syndromes in India. Regular blood transfusions became often the lifeline of the children, which causes serious morbidity and mortality. Hydroxyurea is expected to improve chronic anemia in these children, often at the cost of adverse effects. Data about the safety and efficacy of hydroxyurea in children with E-beta thalassemia is scarce.
Objectives: The present study was aimed to assess the efficacy and safety of hydroxyurea in reducing transfusion requirement in children with E-beta thalassemia, with monitoring of growth parameters. Methods: It was a prospective cohort study done in children with E-beta thalassemia, both Transfusions-Dependent (TDT) and Non-Transfusion-Dependent (NTDT), aged between 5-12 years. All children were treated with hydroxyurea with a dose of 10 mg/kg per day for 12 months, and followed up thereafter for at least 1 year.
Results and Conclusion: A total of 63 children were enrolled in the study. Hydroxyurea causes significant increment of baseline hemoglobin, both in TDT and NTDT. Transfusion requirement was significantly reduced in both the subgroups. There was no impairment of growth parameters during the study. Adverse effects were minimal and self-limiting. Hence the study concludes that, hydroxyurea is an effective and safe drug in children with E-beta thalassemia.
Keywords
Thalassemia, E-beta, Children, Hydroxyurea.
Introduction
Thalassemia syndromes are commonest single gene disorders in the world [1]. Within it, E-beta thalassemia is a common hemolytic anemia especially found in Southeast Asia including India [2]. It can present a varied range of clinical manifestations, both as Transfusion Dependent (TDT) and Non-Transfusion Dependent (NTDT) phenotypes. Whereas blood transfusion is the mainstay of care for patients with thalassemia, however, it comes with the risk of blood-borne infections, iron overload, and adverse reactions that could become a major source of morbidity and mortality.
Hydroxyurea (Hu), an S-phase specific cytotoxic drug, antimetabolic and antineoplastic agent also a fetal Hb inducer, is expected to improve chronic anemia and decrease the need for blood transfusions in patients with thalassemia, by reducing alpha and globin chain imbalance [3]. Mechanisms proposed for the induction of HbF by HU include rapid erythroid regeneration, increased Erythropoietin (EPO) production, apoptosis, Nitric Oxide (NO) production increased guanylate cyclase activity and activation of the p38 MAPK pathway [4].
Though Hu has shown its efficacy in reducing transfusion requirements in adults with E-beta thalassemia it can also be associated with several serious adverse effects. Often it was seen that children had reduced transfusion requirements, at the cost of stunting growth. Studies evaluating the effect of Hu therapy in the pediatric age group and is scarce. Hence this study was done to evaluate the safety and efficacy of Hu in the treatment of patients of E-β Thalassemia with special emphasis on monitoring growth parameters.
Aims and objective
• To assess the efficacy of hydroxyurea in reducing transfusion requirement in patients of E-beta thalassemia, with monitoring of growth parameters.
• To assess the safety of hydroxyurea in patients with E-beta thalassemia.
Material and Methods
Study design and setting
It was a prospective cohort study done in a tertiary care hospital, in Kolkata. Patients with E-beta thalassemia, aged between 5-12 years, who attended either the inpatient or outpatient department of the hospital, were included in this study. Children should have available adequate medical records supplemented with history from reliable sources. Children who were having any other causes of anemia: cardiac, pulmonary, hepatic, or renal dysfunction; hepatitis B, C, or HIV seropositivity were excluded from the study. All procedures performed in studies involving human participants were following the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was registered under memo no. MC/KOL/IEC/242/1-2019 at the institutional research ethics board. The study began after taking written informed consent from the guardian/parents and assent from the patient.
Study details and participants
After taking detailed history including clinical-epidemiological information, and treatment records including blood transfusion-related history, clinical examinations were done to assess the growth parameters (height and weight) according to WHO criteria, the status of hepato-splenomegaly, and other relevant findings. After filling out the detailed medical proforma, children were classified as Transfusion Dependent (TDT) or Non-Transfusion Dependent Thalassemia (NTDT), based on available information.
TDT: Children who required lifelong regular red blood cell transfusions for survival. For the study, it was defined as more than 6 transfusions in the last 6 months [5].
NTDT: Children who didn’t require lifelong regular transfusions for survival. For the study, it was defined as less than 6 transfusions in the last 6 months [5].
Baseline laboratory evaluation includes a complete blood count and HPLC. CBC was done by SYSMEX auto analyzer and HPLC was done by Bio-Rad, VARIANT II β-thalassemia short program reorder pack.
All children were treated with hydroxyurea (Hu) with a dose of 10 mg/kg per day, at least for 12 months. If there is leukopenia (WBC<2000/cmm), the dose of Hu was reduced by 2 mg/kg every week. They were allowed to take blood transfusions as per the standard treatment protocol of TDT and NTDT [6,7]. Data were collected regarding transfusion requirement, growth parameters, complete blood count, and biochemical parameters. HPLC was done every 3 months. Initially, they were followed up every fortnightly for 3 months, followed by a monthly interval during the rest of the study period.
Endpoints
The primary endpoints defined for this study were the decrement of transfusion requirement and increase of hemoglobin level from baseline after treatment with Hu, with no added adverse effect. Secondary endpoints are defined as adequate gain in height and Wt and rise in HbF. Adverse events were evaluated according to Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 published by US National Cancer Institute (NCI)[8,9].
Data collection
Patients were enrolled in this study from February 2019 to January 2020. All the children were followed up to December 2020, i.e. minimal of 1 year. Data were analyzed using statistical methods (mean, standard deviation) and software.
Statistical version 6 (Tulsa, Oklahoma: stat soft Inc., 2001, graph pad prism version 5 (San Diego, California: graph pad software Inc., 2007). The study of significance was analyzed by the chi-square test for qualitative data, fisher’s exact test, 2- tailed ANOVA followed by Tukey’s test between two individual time points, friedman’s ANOVA followed by dunn’s test, student’s unpaired t-test, Wilcoxon’s matched-pairs signed rank test. p-value<0.05 was considered significant.
Result
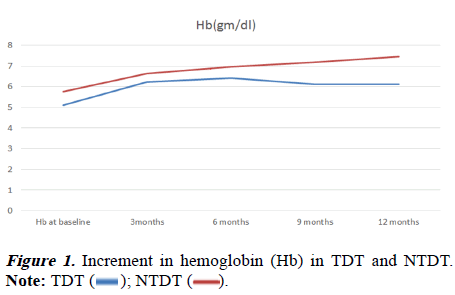
A total of 63 children were enrolled in the study. Out of which 14 children were TDT and 49 children were NTDT. The mean Hb at baseline was 5.62 ± 0.67 gm/dl before Hu therapy. After treatment, it showed increment at every interval, namely 3, 6, 9 and 12 months. At 12 months mean Hb was 7.15 ± 0.81 gm/dl (Table 1).
| No. of patient | Mean | Median | Minimum | Maximum | Std. Dev. | |
|---|---|---|---|---|---|---|
| Baseline Hb(gm/dl) | 63 | 5.62 | 5.7 | 3.8 | 6.9 | 0.671 |
| Hb at 3 m | 63 | 6.55 | 6.5 | 4.9 | 8.7 | 0.583 |
| Hb at 6 m | 63 | 6.85 | 6.9 | 3.4 | 9.2 | 0.817 |
| Hb at 9 m | 63 | 6.94 | 7 | 3.4 | 8.9 | 0.855 |
| Hb at 12 m | 63 | 7.15 | 7.3 | 3.4 | 8.1 | 0.816 |
Table 1. Comparison of Hb during the study.
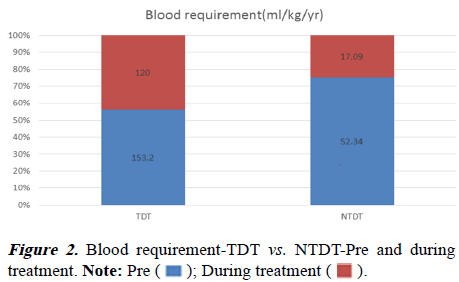
There was more rise of Hb in NTDT (5.77 to 7.45 gm/dl) from baseline in comparison to TDT (5.10 to 6.12 gm/dl). The pvalue was statistically significant when the two groups were compared (Table 2 and Figure 1). The children with TDT have a mean blood requirement of 153.2 ml/kg/year before initiation of treatment. After one year of treatment, it was reduced to 120 ml/kg/year. It was found to be statistically significant between pre and during-treatment blood requirement and the p-value was 0.012.
| Mean TDT | Mean NTDT | t-value | Dosage form | p-value | |
|---|---|---|---|---|---|
| Hb at 0 m | 5.1 | 5.77 | -3.56672 | 61 | 0.001 |
| Hb at 3 m | 6.23 | 6.64 | -2.40865 | 61 | 0.019 |
| Hb at 6 m | 6.42 | 6.97 | -2.30621 | 61 | 0.025 |
| Hb at 9 m | 6.11 | 7.17 | -4.72868 | 61 | 0 |
| Hb at 12 m | 6.12 | 7.45 | -7.25277 | 61 | 0 |
Table 2. Comparison of Hb (gm/dl) between TDT and NTDT.
In the NTDT children, before initiation of therapy mean blood requirement was 52.34 ml/kg/year and after one year of treatment, it was reduced to 17.09 ml/kg/year. It was also found to be statistically significant and the p-value was 0.00 (Table 3 and Figure 2).
| No of patients | 1 year Blood Requirement Pre therapy | 1 year Blood Requirement during treatment | p-value | |
|---|---|---|---|---|
| TDT | 14 | 153.2 | 120 | 0.012 |
| NTDT | 49 | 52.34 | 17.09 | 0 |
Table 3. Comparison between pretreatment and during treatment blood requirement (ml/kg/yr).
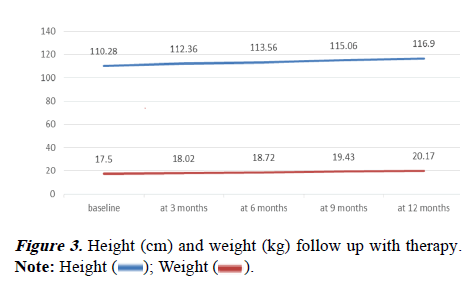
Both TDT and NTDT showed progressively increasing height and weight during the study period. The mean height at baseline TDT was 103.29 cm and NTDT was 112.28 cm. At the end of the follow-up for 1 year mean height for TDT was 109.39 cm and for NTDT was 119.05 cm. The mean Wt at baseline for TDT was 17.83 kg and for NTDT 17.41 kg. The mean weight at 12 months was 20.17 kg for TDT and 20.17 kg for NTDT. There was no significant Ht and Wt difference in follow-up between TDT and NTDT (Table 4 and Figure 3).
| Mean TDT | Mean NTDT | t-value | Dosage form | p-value | |
|---|---|---|---|---|---|
| Baseline Ht (cm) | 103.29 | 112.28 | -1.83374 | 61 | 0.072 |
| Ht at 3 m | 106.08 | 114.03 | -1.57659 | 60 | 0.12 |
| Ht at 6 m | 106.54 | 115.57 | -1.84807 | 61 | 0.069 |
| Ht at 9 m | 107.71 | 117.15 | -1.91961 | 61 | 0.06 |
| Ht at 12 m | 109.39 | 119.05 | -1.94565 | 61 | 0.056 |
| Baseline Wt (kg) | 17.83 | 17.41 | 0.31884 | 61 | 0.751 |
| Wt at 3 m | 18.4 | 17.91 | 0.37104 | 61 | 0.712 |
| Wt at 6 m | 19.05 | 18.62 | 0.32856 | 61 | 0.744 |
| Wt at 9 m | 19.67 | 19.37 | 0.23775 | 61 | 0.813 |
| Wt at 12 m | 20.17 | 20.17 | 0.00156 | 61 | 0.999 |
Table 4. Growth parameters during the study.
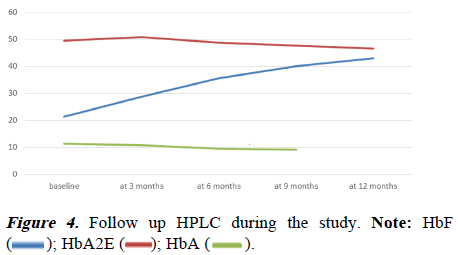
There was also a minimal increment in liver and spleen size during the study period. This concludes that the growth of the children was normal, during the study. Follow-up HPLC showed a progressive increase in Hb F over the months. At 12 months of the study, the mean value of HbF was 42.98 ± 6.99 (Table 5 and Figure 4). Transient leukopenia was seen in 2 patients within 2 weeks of therapy and it was reversible with a reduction of drug dosage. Subsequently, they tolerated the drug very well. No other adverse effects were seen during the study period.
| Valid number of patient | Mean | Median | Minimum | Maximum | Std. Dev. | |
|---|---|---|---|---|---|---|
| HbF at 0 m | 63 | 21.4 | 18.6 | 8 | 43.3 | 8.709 |
| HbF at 3 m | 63 | 28.71 | 28.4 | 18.6 | 44.5 | 6.617 |
| HbF at 6 m | 63 | 35.53 | 34.6 | 22.6 | 52.1 | 6.476 |
| HbF at 9 m | 63 | 39.97 | 39.7 | 23.7 | 54.2 | 6.86 |
| HbF at 12 m | 63 | 42.98 | 43.7 | 25.6 | 56.4 | 6.992 |
Table 5. Follow up of HbF at base line, at 3 months, 6 months, 9 months, and 12 months.
Discussion
In children with E-beta thalassemia, during treatment with Hu, both TDT and NTDT showed a persistent rise of hemoglobin at different points in time, as described in this study. However, there was more rise of mean Hb from baseline in NTDT compared to TDT, which was statistically significant (pvalue< 0.05). This was a unique finding in this study.
Regular blood transfusion is a major obstacle in the life of children with thalassemia. Hydroxyurea significantly reduced the transfusion requirement both in TDT and NTDT as described in this study. All the children tolerated the drug well. Transient leucopenia was observed in only two children, who were also able to continue the medicine at a reduced dosage.
Often, the growth was jeopardized and hepato-splenomegaly was augmented when the transfusion is denied in thalassemic. This was not seen in this study. All the children, while on Hu therapy, both TDT and NTDT, showed gradually increasing Wt at different periods. Mean height increments over time during treatment were found to be statistically significant (pvalue< 0.05). There was no significant height increment difference between TDT and NTDT.
Conclusion
There was a decrement in the size of the liver and spleen during the study period. Hence the study concludes that, in children with E-beta thalassemia, treated with hydroxyurea, there was a reduction in transfusion requirement and an increase in baseline hemoglobin. Hydroxyurea reduces transfusion requirement and increases baseline hemoglobin in children with E-beta thalassemia, both transfusion dependent and non-transfusion dependent, without hampering growth parameters with no added adverse effect.
 ); NTDT (
); NTDT ( ).
).
 ); During treatment (
); During treatment ( ).
).
 ); Weight (
); Weight ( ).
).
 ); HbA2E (
); HbA2E ( ); HbA (
); HbA ( ).
).