Current Pediatric Research
International Journal of Pediatrics
Effect of iron deficiency anemia on simple and complex febrile seizures in children in Karbala province.
Sabah Hassan Alatwani1, Abdul Kareem A Jasim1, Mustafa Muayad Sahib2, Alaa Jumaah Nasrawi1*
1Department of Pediatrics, College of Medicine, University of Karbala, Najaf, Iraq
2Department of Pediatrics, College of Medicine, Pediatric Teaching Hospital of Karbala, Najaf, Iraq
- Corresponding Author:
- Alaa Jumaah Nasrawi
Department of Pediatrics
University of Kufa
College of Medicine
Najaf, Iraq
E-mail: alaaj.nasrawi@uokufa.edu.iq
Accepted date: August 23th, 2021
Background: Febrile convulsion is the most common central nervous system disease seen in children. There are hypotheses that threshold of neuron excitation may be affected by iron deficiency anemia. Effect of Iron Deficiency Anemia (IDA) on febrile seizures and recurrent febrile seizure is investigated. Materials and Methods: Across sectional study was conducted among 121 children with febrile convulsion who divided into 2 groups: 70 with simple febrile seizure and 51 with complex febrile seizure. Results: No significant differences had been found between both groups in demographic variables. Analysis of blood showed that low levels of Hemoglobin (HB), Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH) and S. ferritin levels and high Red Cell Distribution Width (RDW) were more frequent in complex group than simple group at P value<0.05. Iron deficiency anemia was found in 24% of all patients. Recurrence of febrile seizure was 52.1% of all patient, was more likely to occur in IDA patients than those with no IDA in about 2.5 folds, (OR=2.53, Pvalue= 0.037). Conclusion: Febrile seizures associated with iron deficiency anemia. Complex febrile seizure is more frequent with iron deficiency anemia than in those with simple febrile seizures. Febrile seizures are significantly associated with higher recurrence in patients with iron deficiency anemia.
Keywords
Iron, Deficiency, Febrile, Seizure.
Introduction
Febrile Seizures (FS) are the most common cause of convulsions in children and a frequent cause of emergency hospital admissions. Between 2% and 5% of children (more common in boys) in Europe and the United States experience at least one FS before the age of 5 years [1-3]. Although earlier Indian studies suggested that up to 10% of children experience a febrile seizure, recent data indicate that the incidence rate in India is similar to western figures [4,5]. The pathophysiology of febrile seizures remains unclear. It is generally believed that it is an age dependent response of the immature brain to fever. This postulation is supported by the fact that most (80%-85%) febrile seizures occur between 6 months and 5 years of age, with the peak incidence at 18 months [1-2,6]. Simple febrile seizures are those that last less than 15 minutes and are generalized (tonic-clonic). Complex febrile seizures are prolonged more than 15 minutes, focal, or multiple within 24 hours [7].
Despite the abundance iron in the environment, iron deficiency is the most common nutritional deficiency in the western world and the most common cause for anemia worldwide. The iron status of infants and children is especially precarious because of exaggerated needs imposed by growth. The national family health survey III documented that about 78.9% children between the ages of 6-35 months were anemic [8]. Iron is used as cofactor for metabolism of many neurotransmitters, monoamine and aldehyde oxidase in the brain [9]. The metabolism of this neurotransmitter will be affected in the patient with iron deficiency leading to decrease in this neurotransmitter, which may decrease the threshold for seizure [10]. Fever can worsen the negative effects of low serum ferritin on the brain and trigger seizure. Effect of iron deficiency anemia on simple and complex types of febrile seizure and on recurrent febrile seizure is investigated in this study.
Methods
Patients
Across sectional study was conducted among 121 children with febrile convulsion in Karbala pediatric teaching hospital during the period from 1 March to 1 December 2018 in Karbala province. Patients were divided into two groups. The first group included 70 children with Simple Febrile Seizure (SFS) that determined based on criteria of tonic-clonic, lasting fever for 15 mins, and no recurrent within a 24 hours. The second group included 51 children with Complex Febrile Seizure (CFS) that determined based on criteria of prolong FS to more than 15 mins, focal, and can recurrent within 24 hours. Blood sample was collected from all patients for measurement Hemoglobin (HB), Red Blood Cells (RBCS), Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), Red cell Distribution Width (RDW), and serum ferritin level.
Data were collected from parents of patients by questionnaire design to determine the age, temperature, and family history of febrile seizure or epilepsy. The age of children was ranged from 6 to 60 months (mean age: 33). The sample was taken according to the definition and criteria of febrile seizures are: seizures that occur between the age of 6 and 60 months with a temperature of 38°C or higher that are not the result of central nervous system infection or any metabolic imbalance, and that occur in the absence of a history of prior a febrile seizures.
Iron deficiency anemia was defined based on WHO criteria for age when HB<11 g/dl, MCH less than 26 pg (normal range 26-32 pg), MCV less than 70 fL (normal range; 70-96 fL), RDW more than 15% (normal range; 11.5-14.5), decrease red blood cell mass (normal range; 4.06 to 5.30 × 106/UL), serum ferritin less than 30 ng\ml (normal range; 30-400 ng\ml). Patients with CNS infection like meningitis or encephalitis, metabolic disease, or epilepsy were excluded from the study.
Ethical and administrative approval
Ethical approval on study conduction obtained from Iraqi ministry of health, Karbala training centre of the Arab board for health specialization and from Karbala health directorate. An oral consent was taken from each parentprior to the interview, with short explanation on the objectives of study.
Statistical analysis
Data were entered and analyzed using the statistical package for social sciences version 24, variables presented as frequencies percentages, median, mean and standard deviation. Chi square test used to assess the significance between studied groups. Student t test used to compare means. Binary regression analysis performed to control the possible effect of some variables on the association between IDA and recurrence. Odds Ratio (OR) which is an estimator of an association between a risk and outcome, it was calculated according to standard equation, OR of more than one indicated the more likelihood that an event present in a group rather than the other group, while if it is less than one, it indicates the less likelihood. OR of one indicates no difference between both groups in regard to presence of a factor under study. Level of significance set at p value ≤ 0.05 to be significant.
Results
The median age was 19 months in simple group and 24 months in complex group. Moreover, the age distribution into 4 age subgroups with a 12 months interval revealed no statistical significant differences between both groups, P-value>0.05. The gender distribution in this study revealed that male more than female have febrile seizure, age between (13-24) months have more febrile seizure than other age subgroups (Table 1).
| Variable | Patient group | Total No. | P value | ||||
|---|---|---|---|---|---|---|---|
| Simple (N=70) | Complex (N=51) | ||||||
| No. | % | No. | % | ||||
| Age (month)* | 6-12 | 16 | 22.9 | 11 | 21.6 | 27 | 0.98 |
| 13-24 | 26 | 37.1 | 18 | 35.3 | 44 | ||
| 25-36 | 16 | 22.9 | 12 | 23.5 | 28 | ||
| 36-60 | 12 | 17.1 | 10 | 19.6 | 22 | ||
| Total | 70 | 57.9 | 51 | 42.1 | 121 | ||
| Median age (IQR*) | 19 (13-33) | 24 (13-30) | |||||
| Gender | Male | 43 | 61.4 | 29 | 56.9 | 72 | 0.61 |
| Female | 27 | 38.6 | 22 | 43.1 | 49 | ||
| Total | 70 | 57.9 | 51 | 42.1 | 121 | ||
Table 1. Age and gender distribution of the studied groups. IQR: Inerquartile Range.
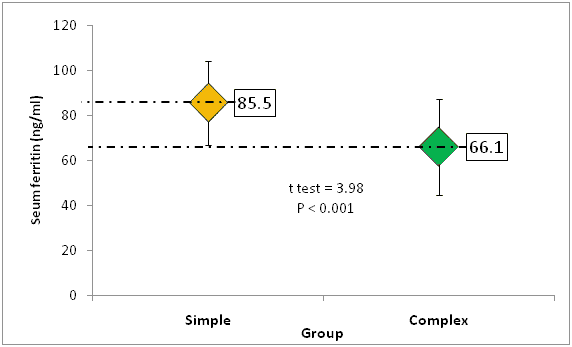
The mean RBCs count was 4.4+0.42 RCC × 106 cells/ml and 4.2 ± 0.39 × 106 cells/ml. The mean Hemoglobin (HB) level was 10.9 ± 0.82 and 10.5 ± 0.77 in simple and complex groups, respectively. Additionally, in simple group the mean values of MCV, MCH, and RDW were 73.0 femtoliter, 24.9 pg/cell and 15.3% , respectively and the corresponding levels in complex group were 66.5%, 23.2% and 16.4%, respectively, with statistically significant differences in these parameters between both groups (P<0.05) (Table 2). Furthermore, low HB, MCV, MCH and serum ferritin levels were more frequent in complex group than simple group in all comparisons, P-value<0.05 (Table 3). On the other hand, the mean serum ferritin was significantly higher in simple than complex fit group, 85.5 ng/ml vs. 66.1 ng/ml, respectively (P-value<0.001) (Figure 1).
| Parameter | Group | P value | |
|---|---|---|---|
| Simple (N=70) | Complex (N=51) | ||
| RBCs × 106 cell/ml | 4.4 ± 0.42 | 4.2 ± 0.39 | 0.072 |
| Hb (g/dL) | 10.9 ± 0.82 | 10.5 ± 0.77 | 0.015 |
| MCV femtoliter | 73.0 ± 9.7 | 66.5 ± 16.5 | 0.007 |
| MCH pg/cell | 24.9 ± 3.3 | 23.2 ± 3.9 | 0.019 |
| RDW% | 15.3 ± 2.2 | 16.4 ± 2.7 | 0.024 |
| Serum ferritin (ng/ml) | 85.5 ± 24.8 | 66.1 ± 18.7 | <0.001 |
Table 2. Hematological parameters of the studied group.
| Parameter | Group | P value | ||||
|---|---|---|---|---|---|---|
| Simple (N=70) | Complex (N=51) | |||||
| No. | % | No. | % | |||
| Hb | Low | 33 | 47.1 | 35 | 68.6 | 0.019 |
| Normal | 37 | 52.9 | 16 | 31.4 | ||
| MCV | Low | 36 | 51.4 | 37 | 72.5 | 0.014 |
| Normal | 34 | 48.6 | 14 | 27.5 | ||
| MCH | Low | 42 | 60 | 41 | 80.4 | 0.017 |
| Normal | 28 | 40 | 10 | 19.6 | ||
| Serum ferritin | Low | 12 | 17.1 | 18 | 35.3 | 0.022 |
| Normal | 58 | 82.9 | 33 | 64.7 | ||
Table 3. Comparison of levels of HB, MCV, MCH and serum ferritin according to standard reference ranges for diagnosis of IDA.
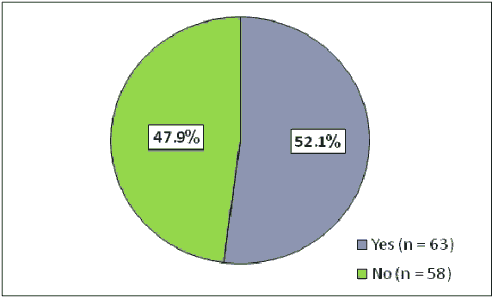
According to the standard reference levels for the diagnosis of IDA, 11 patients (15.7%) in simple group had IDA compared to 18 (35.3%) patients in complex group, according to the value of odds ratio, patients with complex febrile fit were about 1.7 folds more likely to have IDA than those with simple febrile fit, (OR=1.73, P value=0.013) (Table 4). In total, IDA was found in 29 patients represented 24% of the 121 patients (Figure 2). Regarding the recurrence was reported in 63 out of the 121 patients giving a recurrence rate of 52.1%, (Figure 3).
| Diagnosis | Group | Total | ||||
|---|---|---|---|---|---|---|
| Simple (N=70) | Complex (N=51) | |||||
| No. | % | No. | % | No. | % | |
| IDA | 11 | 15.70% | 18 | 35.30% | 29 | 24 |
| No IDA | 59 | 84.30% | 33 | 64.70% | 92 | 76 |
| Total | 70 | 57.90% | 51 | 42.10% | 121 | 100 |
Table 4. Comparison of frequencies of IDA among studied groups. Odds ratio=1.73 (95% CI: 1.17-2.57), more likely in complex group. P-value=0.013 (significant).
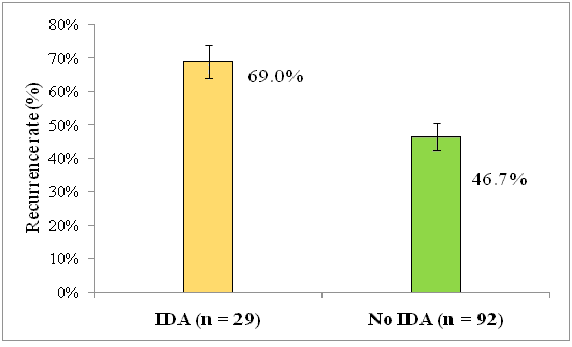
Further analysis was performed to assess the relationship between IDA and recurrence using the cross tabulation between IDA and recurrence. The results of this analysis are shown in Table 5, where recurrence reported in 20 IDA patients out of the 29 giving a recurrence rate of 69% among this subgroup compared to 43 (46.7%) out of the 92 with no IDA, which indicated a significant association between IDA and recurrence. The recurrence was more likely to occur in IDA patients than those with no IDA in about 2.5 folds (OR=2.53, P-value=0.037). Furthermore, the recurrence rates are compared in Figure 4. Additionally, binary regression analysis revealed that the relationship between IDA and recurrence was still significant (P<0.05) after adjustment for the other variables (Table 6).
| Recurrence | IDA | No IDA | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Yes | 20 | 69 | 43 | 46.7 |
| No | 9 | 31 | 49 | 53.3 |
| Total | 29 | 24 | 92 | 76 |
Table 5. Relationship between IDA and recurrence among the total patients (N=121). Odds ratio=2.53 (95% CI: 1.10-6.15), more likely in IDA patients. P-value=0.037 (significant).
| Variables in the regression equation | OR | Significant |
|---|---|---|
| Age | 1.13 | 0.83 |
| Gender | 1.63 | 0.23 |
| Family history of epilepsy | 1.49 | 0.43 |
| Family history of febrile fit | 0.97 | 0.96 |
| IDA | 2.49 | 0.007 |
| Type of febrile fit (complex) | 1.36 | 0.36 |
Table 6. Results of binary regression analysis for the relationship between recurrence and other variables.
Discussion
The risk of febrile seizure is associated with many factors, and many authors are in favor of a multifactorial model [11]. In the literature reviewed there are some publications involving anemia as a risk factor for febrile seizure in children. Pisacane et al. reported that the anemia was the most common underlying pathology in children younger than 2 years with febrile convulsion [12]. Additionally, Kwak et al. found that IDA was associated with increased risk febrile seizures [13]. This suggests a possible association between low levels of iron and the presence of febrile seizure. The explanation of that the threshold of neuron excitation may be affected by iron deficiency anemia.
From the scientific point of view it is important that in establishing the relationship between anemia and febrile seizure be counted a new factor for developing this disease, which is easily treatable. Also, perfects the skills in the diagnostic evaluation and therapeutic course of this disease. Therefore, the present study tried to assess the relationship between Iron Deficiency Anemia (IDA) and febrile convulsions in both simple and complex subtypes among group of Iraqi children presented to our hospital.
In the present study, the median age was 19 months and 24 months in simple and complex groups, respectively, furthermore, the subgrouping of patients into 4 age groups, indicated that almost two thirds of the patients at the age of two years and below. The gender distribution revealed the dominance of males in both groups, however, no statistically significant differences had been found between both studied group neither in age nor the gender. The age and gender distribution of the patients in this study is consistent with epidemiological and clinical picture of the febrile convulsions that reported in previous literatures, which were documented in this study the incidence of febrile seizures is relatively higher in younger children that age between 13 months-24 months and in males more than female.
According to the standard criteria for the diagnosis of IDA, the present study found 29 cases (24%) had IDA, 18 in complex group and 11 in simple group. These findings indicated two points; the first that IDA is not uncommon in children with febrile convulsions compared to general population and the second point that IDA was more frequent in children with complex than simple febrile fit with almost 1.7 folds. Children with complex febrile seizures were more likely to have IDA compared to those with simple febrile seizures. These findings agreed that reported in previous studies. Eda et al. found iron deficiency anemia is more frequently seen among patients with complex febrile convulsion than in patients with simple febrile convulsion [14]. Also, Hartfield et al. found that patients with complex seizures had lower HB levels and more likely to have IDA compared to those with simple seizures. Other findings by Hatfield that IDA patients were almost twice likely to found in children with febrile seizures than those with febrile illness without seizures [15]. Moreover, systemic review conducted by Jun et al. concluded that IDA ratio was higher in complex febrile seizure than simple febrile seizure but not statistically significant [16].
The explanation of complex febrile seizures affected by IDA more than simple febrile convulsion is that complex febrile seizure is a major risk factor of epilepsy and naturally more inflammatory changes occur in the brain than simple febrile seizure [14]. Thus, effect of iron deficiency more in complex febrile seizure because iron is used as cofactor for metabolism of many neurotransmitters, monoamine and aldehyde oxidase inthe brain [9]. The metabolism of this neurotransmitter will be affected in the patient with iron deficiency leading to decrease in this neurotransmitter, which may decrease the threshold for seizure [10]. From other point of view, Kumari et al stated that IDA is a significant risk factor for simple febrile seizures in children aged 6 months to 3 years. However, Kumari compared his cases with a control group having febrile illness without seizures and did not included cases with complex seizure [17].
From other point of view, lower serum ferritin levels were also reported to be associated with febrile convulsion. Daoud et al. found that a significant decrease in the plasma ferritin levels was associated with higher risk of first febrile seizure in children under age of 4 compared with a reference group [18]. Furthermore, other studies conducted by [19]. Momen et al. also supported the findings of Daoud et al., that IDA and lower S ferritin level was associated with higher incidence of febrile seizures, and suggested that Iron insufficiency have an important role in FS [18,20]. However, these studies compared their findings with control groups and did not compare between simple and complex febrile convulsions subgroups.
Conversely, two previous studies found no significant association between IDA and Febrile Seizures. An Iranian study was conducted by Amirsalari et al. [21] and the other study was conducted by Kobrinsky et al. reported that IDA was less likely to occur in children with febrile seizures compared to controls [22]. The explanation for the differences in the findings between these two studies and our study could be attributed to the geographical variation and the prevalence of anemia in different populations where the relationship appear to be more significant in areas with low to moderate prevalence of anemia but not in those with high prevalence.
The present study the recurrence of febrile seizure occurred in 52.1% of the cases. These findings are close to that of some previous earlier studies. Boonluksiri found that the risk of recurrence of febrile seizure was as high as 68% [23]. Lower recurrence rates were reported by other studies; Sogawa et al. found the recurrence less than 20% [24]. Sogawa et al. suggested that remote symptomatic etiology and abnormal EEG are important risk factors for recurrence [24].
The current study found that recurrence was significantly associated with IDA, where cases with IDA were about 2.5 folds more likely to have recurrence of FS than those with no IDA. Similarly, Dawn et al. documented that iron deficiency increases the recurrence febrile seizure and therapy for iron deficiency decrease the risk of febrile seizure recurrence [25]. Also Papageorgiou et al. found iron deficiency seems to be related to the pathogenesis of FS, early prevention and detection could reduce the frequency of FS. Therefore, iron status work-up could be established as a routine screening for all children who are in high risk to have FS or for preventing a recurrence [26]. Explanation for effect of IDA on recurrence of FS is that iron deficiency effect on seizure threshold and may also effect on type, duration and recurrence [12]. Unfortunately, very few studies focus the light on the association between IDA and the recurrence of febrile seizure. This is a strength point to the present study that tried makes this point under debate. Moreover, the present study assess the association between recurrence and IDA after adjustment for the effect of other variables like age, gender, family history of febrile seizure or epilepsy and EEG, because multiple factors could possibly related to increase the recurrence rate.
Conclusion
Febrile seizures are associated with Iron deficiency anemia. Complex febrile seizure more frequent with Iron deficiency anemia than in those with simple febrile seizures. Febrile seizures were significantly associated with higher recurrence in patients with iron deficiency anemia.
References
- Hackett R, Hackett L, Bhakta P. Febrile seizures in a South Indian district: Incidence and associations. Dev Med Child Neurol 2007; 39(6): 380-4.
- Gourie-Devi M, Gururaj G, Satishchandra P, et al. Prevalence of neurological disorders in Bangalore, India: A community-based study with a comparison between urban and rural areas. Neuroepidemiology 2004; 23(6): 261-8.
- Berg AT. Are febrile seizures provoked by a rapid rise in temperature? Am J Dis Child 2013; 147(10): 1101-3.
- Rantala H, Uhari M, Hietala J. Factors triggering the first febrile seizure. Acta Paediatr 2005; 84(4): 407-10.
- Berg AT, Shinnar S, Shapiro ED, et al. Risk factors for a first febrile seizure: A matched case-control study. Epilepsia 1995; 36(4): 334-41.
- Sansone R, Impagliazzp N, Coppola A, et al. Iron deficiency anemia and febrile convulsion; case control study in children under 2 years. BMJ 1996; 313(7053): 343.
- Kliegman R, Stanton B, St Geme JW, et al. (2016) Nelson textbook of pediatrics. 20th edn, Pennsylvania: Elsevier, Philadelphia.
- Wheby MS, Jones LG, Crosby WH. Studies on iron absorption: Intestinal regulatory mechanisms. J Clin Invest 1964; 43(7): 1433-42.
- Gardner JW, Dinsmore RC. Evolution of the concept of the febrile seizure as it developed in the American medical literature 1800-1990. J Hist Med Allied Sci 1995; 50(3): 340-63.
- Mittal R, Marwaha N, Basu S, et al. Evaluation of iron stores in blood donors by serum ferritin. Indian J Med Res 2006; 124(6): 641-6.
- Delpisheh A, Veisani Y, Sayehmiri K FA. Febrile seizures: Etiology, prevalence, and geographical variation. Iran J Child Neurol. 2014; 8(3): 30-7.
- Pisacane A, Sansone R, Impagliazzo N, et al. Iron deficiency anaemia and febrile convulsions: Case-control study in children under 2 years. Br Med J 1996; 313(7053): 343-4.
- Kwak BO, Kim K, Kim SN, et al. Relationship between iron deficiency anemia and febrile seizures in children: A systematic review and meta-analysis. Seizure 2017; 52: 27-34.
- OzaydIn E, Arhan E, Cetinkaya B, et al. Differences in iron deficiency anemia and mean platelet volume between children with simple and complex febrile seizure. Seizure 2012; 21(3): 211-4.
- Hartfield DS, Tan J, Yager JY, et al. The association between iron deficiency and febrile seizures in childhood. Clin Pediatr (Phila) 2009; 48(4): 420-6.
- Jun YS, Bang HI, Yu ST, et al. Relationship between iron deficiency anemia and febrile convulsion in infant. Korean J Pediatr 2010; 53: 392-6.
- Kumari PL, Nair MKC, Nair SM, et al. Iron deficiency as a risk factor for simple febrile seizures-a case control study. Indian Pediatr 2012; 49(1): 17-9.
- Daoud AS, Batieha A, Abu-Ekteish F, et al. Iron status: A possible risk factor for the first febrile seizure. Epilepsia 2002; 43(7): 740-3.
- Vaswani RK, Dharaskar PG, Kulkarni S, et al. Iron deficiency as a risk factor for first febrile seizure. Indian Pediatr 2010; 47(5): 437-9.
- Momen AA, Nikfar R, Karimi B. Evaluation of iron status in 9-month to 5-year-old children with febrile seizures: A case control study in the south west of Iran. Iran J Child Neurology 2010; 4(2): 45-50.
- Amirsalari S, Doust ZTK, Ahmadi M, et al. Relationship between iron deficiency anemia and febrile seizures. Iran J Child Neurol 2010; 4(1): 27-30.
- Kobrinsky NL, Yager JY, Cheang MS, et al. Does iron deficiency raise the seizure threshold?. J Child Neurol 1995; 10(2): 105-9.
- Boonluksiri P. Risk of recurrence following a first unprovoked seizure in Thai children. Neurol J Southeast Asia 2003; 8: 25-9.
- Sogawa Y, Maytal J. Emergency department admission of children with unprovoked seizure: Recurrence within 24 hours. Pediatr Neurol 2006; 35(2): 98-101.
- Hartfield DS, Tan J, Yager JY, et al. association between iron deficiency anemia and febrile seizures in childhood. Clinpediatr (philla) 2009; 48(4): 420-6.
- Papageorgiou V, Vargiami E, Kontopoulos E, et al. Association between iron deficiency and febrile seizures. Eur J Paediatr Neurol 2015; 19(5): 591-6.