Current Pediatric Research
International Journal of Pediatrics
Clinico-pathological predictors of colonic eosinophilia in childhood: A single-center study.
Mohammad Naghi1*, Khaled Zalata2, Khadega Ali2, Khaleel Wafi2, Magdy Ibrahim3, Ahmed Abdullah4
1Department of Pediatrics and Medicine, Helwan University, Helwan, Egypt
2Department of Pathology and Medicine, Mansoura University, Mansoura, Egypt
3Department of Obstetrics and Gynecology, Research and Biostatistics Unit, Cairo University, Giza, Egypt
4Department of Pediatrics and Medicine, Mansoura University, Mansoura, Egypt
- Corresponding Author:
- Mohammad Naghi
Department of Pediatrics and Medicine,
Helwan University, Helwan,
Egypt
E-mail: m.naghi@med.helwan.edu.eg
Received: 26 January, 2022, Manuscript No. AAJCP-23- 87508; Editor assigned: 30 January, 2022, Pre QC No. AAJCP-23-87508(PQ); Reviewed: 06 February, 2023, QC No. AAJCP-23-87508; Revised: 17 January, 2023, Manuscript No. AAJCP-23-87508(R); Published: 28 February, 2023, DOI:10.35841/0971-9032.27.02.1780-11786.
Objectives: Childhood colonic eosinophilia is a perplexing outcome with a broad differential. The practitioner has little guidance on how to approach these patients. We examined individuals with colonic eosinophilia and studied the clinic-pathological parameters correlated with their diagnoses. We aimed to see if we could find predictors of colonic eosinophilia without colonoscopy and if these predictors could be used for monitoring. This was achieved so that a colonoscopy would not have to be used unless there was doubt about the diagnosis.
Methods: In order to compare children with colonic eosinophilia (N=56) in their histopathology to controls without the condition (N=120), we conducted a 10-year retrospective chart review.
Results: Individuals with colonic eosinophilia had significant clinical and laboratory characteristics contrasted to controls (P<0.001). Age, platelet count, and fecal calprotectin were the three factors that might be considered predictors in diagnosing Colonic Eosinophilia.
Conclusion: Age, platelet count, and calprotectin were good predictors for colonic eosinophilia cases. Such predictors could be of value in monitoring patients.
Keywords
Colitis, Eosinophil, Eosinophilic colitis, Colonic eosinophilia.
Introduction
Childhood Eosinophilic Gastrointestinal Disorders (EGIDs) are uncommon chronic inflammatory abnormalities of the digestive tract with unidentified lengthy consequences [1]. The symptom is ascertained by the site in GI, also implicated to the eosinophilic inflammation's degree and depth via the intestine wall. After ruling out a secondary causation of inflammation or a systemic disease in the absence of biological markers, the diagnosis is based on clinical signs and histopathological findings of eosinophilic inflammation, which can be difficult given the lack of rigid histological criteria for EGID (beyond the esophagus) diagnosis [2,3].
In some pediatric patients being evaluated for prevalent gastrointestinal complaints, colonic eosinophilia is a complicated histological finding. The eosinophils' exitance in the colon wall's inflammatory infiltrate leads to the diagnosis. There are few pathological recommendations that distinguish normal from abnormal colonic eosinophilia statistics. In the cecum, the mean extreme eosinophils per High Powered Field (eos/HPF) in normal children varied from 14 to 474 [4-7], and geographic variation have been defined as well [8]. In the lack of a clear cause, including bone marrow transplant, drug reaction, parasitic infection, radiation treatment, constipation, or collagen vascular disease, little is recognized about this finding's prognosis or pathogenesis [9].
The clinical finding demonstrates that colonic eosinophilia is a subtype of Inflammatory Bowel Diseases (IBD) [10-13]. Further researchers define colonic eosinophilia as previous 11 or overlapping [14] the IBD diagnosis, but there is little evidence for this [15]. Colonic Eosinophilia (EC) has also been noted in cases with colonic eosinophilia that do not have IBD or any other recognized etiology. Because they can reveal similar symptoms, distinguishing EC from IBD can be difficult. Previous research has not identified clinicopathologic factors that may aid clinicians in distinguishing between these diseases' various groups. To fully comprehend the colonic eosinophilia's clinical significance, we did a children's retrospective analysis shown at a single center with a pathological diagnosis of "colonic eosinophilia." These study's goals were to: 1) Contrast control colon biopsies to individuals with colonic eosinophilia in order to better describe colonic eosinophilia, 2) Define clinicolaboratory variables that may aid in identifying patients with EC, and 3) Determine whether colonoscopy would be demonstrated to clarify the diagnoses.
Literature
Our study's primary aim was to help pediatric gastroenterologists find predictors for colonic eosinophilia without relying on colonoscopy, which is a little bit invasive. The second aim was to determine if such predictors could be of value for monitoring the patient's response to treatment.
Materials and Methods
Subject selection
We reviewed all patients' medical records aged 1 to 17 years referred to our center, which specializes in evaluating colonic disorders, over the last 12 years from 2008 to 2020.
In Mansoura pediatric gastroenterology center, data including laboratory values, physician notes, and pathology reports were revised. We looked up "eosinophilic colitis," "colonic eosinophilia," "elevated eosinophils," or "eosinophilia."
Because there is no consensus on what constitutes normal against eosinophils' abnormal numbers in the pediatric colon, we didn't utilize eosinophils' particular cut-off number to recruit participants. Rather, we looked at all records in which one of the above terms was used to describe the pathologist's interpretation. A diagnostic threshold of 20 eosinophils HPF was applied to separate significant colonic eosinophilia from control subjects [16]. "Colonic eosinophilia" was diagnosed in this cohort.
Control subjects were selected as having colonic symptoms but with no evidence of eosinophils in their pathology reports. No evidence was found that elevated inflammatory markers (ESR and CRP), anemia, or medications history that might affect colon eosinophil numbers (5-aminosalicylates, systemic steroids, immunomodulators, antibiotics, or biologic therapies). Clinical characteristics of cases associated with colonic eosinophilia and control subjects were identified by reviewing medical records, involving laboratory testing, symptoms, endoscopic outcomes, demographics, and final diagnoses.
Following the initial colonoscopy, cases' laboratory specimens were taken under strict aseptic conditions for the ensuing workup: Complete blood count, Erythrocyte Sedimentation Rate (ESR), and fecal calprotectin level. Complete Blood Count (CBC), including platelet count and indices, was carried out by a fully automated cell counter. Platelets count below 400.000 was considered normal, while more than 400.000 were considered high. ESR below 40 was considered normal, and higher than 40 was considered high. Fecal calprotectin level more than 200 was considered high [17]. Exclusion criteria were: 1) Bone marrow transplant, 2) Under 1 year of age at initial biopsy-diagnoses of allergic colitis, or 3) No calprotectin test performed.
Specimen histological assessments
Three pathologists (KZ, KA, and KW) evaluated all hematoxylin and eosin (H&E) stained colonic tissue sections from "colonic eosinophilia" cases as well as control subjects in the same way. A diagnostic threshold of 20 eosinophils per HPF was applied to separate significant colonic eosinophilia from control subjects [16]. The entire specimen was examined, and the area with the greatest eosinophil density was chosen to count the maximum number of eosinophil per High-Powered Field (eos/HPF) at an amplification of 40. The pathologists (KZ and KA) identified chronic inflammation's signs on histopathology when there was distorted gland architecture, left colon paneth cells, multiple branched glands, or fibrosis. Cryptitis, crypt abscess formation, granulomas and ulceration were searched for in all cases and were reported as positive or negative.
Statistical analysis
When suitable, data were statistically defined in aspects of mean, standard deviation (± SD), range, and median, or percentages and frequencies (cases' number). Kolmogorov Smirnov test was utilized to test numerical data for the normal assumption. Mann-Whitney U-test for independent specimens contrasting two groups and Kruskal Wallis test for comparing more than two groups were utilized to compare numerical variables between study groups. For contrasting categorical data, chi-square (χ2) test was conducted. The exact test was utilized when the anticipated frequency was less than 5.
The significant independent predictors of colonic eosinophilia were identified using multivariate logistic regression analysis. To describe accuracy, researchers used the definitions sensitivity, specificity, +ve predictive value, -ve predictive value, and accuracy rate of markers in diagnosing colonic eosinophilia. Two-sided p-values less than 0.05 were regarded statistically significant. The IBM SPSS (Statistical Package for the Social Science; IBM Corp., Armonk, NY, USA) release 22 for Microsoft windows was utilized for all statistics.
Results
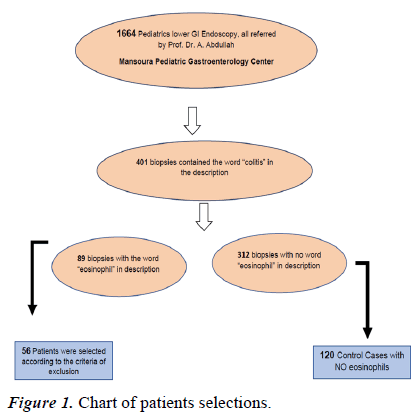
Retrospective analyses to identify cases and determine controls; were conducted utilizing two independent methods, as outlined by the flow chart depicted in Figure 1. 1664 lower GI endoscopy, 401 colitis in pathology description, and eighty-nine patients with colonic eosinophilia were identified. Thirty-three were not included, dependent on exclusion criteria. The number of final selected cohort cases was 56. One hundred twenty controls were chosen from 312 non-eosinophilic patients' data.
As per Table 1, the cases were significantly younger at the time of the first colonoscopy and had a history of asthma, environmental allergies, family allergy history, and/or gastrointestinal disease. Weight loss, high platelet count, high ESR, higher calprotectin level, cryptitis, and the existence of crypt abscess were significantly higher in cases of CE vs. non- CE.
| Variables | EC (n=56) | Non-EC (n=120) | P-value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age at 1st colonoscopy | 3.21 | 2.52 | 6.49 | 3.24 | <0.001 |
| N | % | N | % | ||
| Env/food/drug allergies | 49 | 87.50% | 80 | 66.70% | 0.004 |
| FH of GI illness | 40 | 71.40% | 79 | 65.80% | 0.46 |
| Asthma | 25 | 44.60% | 60 | 50.00% | 0.508 |
| Males | 33 | 58.90% | 71 | 59.20% | 0.976 |
| Hematochezia | 32 | 57.10% | 50 | 41.70% | 0.055 |
| Vomiting | 23 | 41.10% | 41 | 34.20% | 0.375 |
| Diarrhea | 28 | 50.00% | 47 | 39.20% | 0.176 |
| Abdominal pain | 56 | 100.00% | 120 | 100.00% | --- |
| Weight loss | 6 | 10.70% | 0 | 0.00% | 0.001 |
| High platelet count | 41 | 73.20% | 18 | 15.00% | <0.001 |
| High Calprotectin | 43 | 76.80% | 25 | 20.80% | <0.001 |
| High ESR | 35 | 62.50% | 32 | 26.70% | <0.001 |
| Cryptitis | 34 | 60.70% | 28 | 23.30% | <0.001 |
| Crypt Abcess | 31 | 55.40% | 17 | 14.20% | <0.001 |
| IBD | 17 | 30.40% | 28 | 23.30% | 0.32 |
| Ulcer | 1 | 1.80% | 14 | 11.70% | 0.039 |
| Granuloma | 11 | 19.60% | 7 | 5.80% | 0.005 |
Table 1. Patient demographics and Clinco-laboratory factors comparing colonic eosinophilia cases to those with control (noneosinophilic) biopsies.
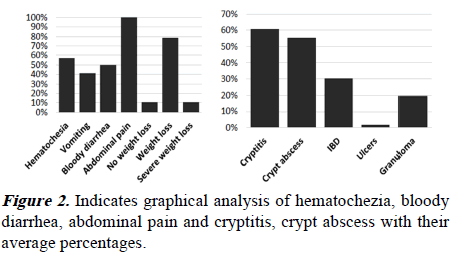
We found that hematochezia, bloody diarrhea, abdominal pain, and weight loss are highly frequent cases of CE, as shown in Figure 2A. Cryptitis and crypt abscess are highly frequent in cases of CE, as revealed in Figure 2B.
Table 2 shows the results of the univariate analysis. We found that age, weight loss, high platelet count, high calprotectin level, higher ESR levels, presence of cryptitis, and crypt abscess in pathology were highly significant in cases compared to controls.
| Variables | EC (n=56) | Non-EC (n=120) | OR | P-value | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Age | ||||||
| 6 years or more | 9 | 16.1 | 65 | 54.2 | 0.162 | <0.001 |
| <6 years | 47 | 83.9 | 55 | 45.8 | 6.172 | |
| Sex | ||||||
| Male | 33 | 58.9 | 71 | 59.2 | 0.99 | 0.976 |
| Female | 23 | 41.1 | 49 | 40.8 | 1.01 | |
| Abdominal pain | 56 | 100 | 120 | 100 | ||
| Diarrhea | 28 | 50 | 47 | 39.2 | 1.553 | 0.176 |
| Weight loss/poor gain | 6 | 10.7 | 0 | 0 | 0.001 | |
| High PLT count (>400 × 103) | 41 | 73.2 | 18 | 15 | 15.489 | <0.001 |
| High calprotectin (>200) | 43 | 76.8 | 25 | 20.8 | 12.569 | <0.001 |
| High ESR | 35 | 62.5 | 32 | 26.7 | 4.583 | <0.001 |
| IBD | 17 | 30.4 | 28 | 23.3 | 1.432 | 0.32 |
| Cryptitis | 34 | 60.7 | 28 | 23.3 | 5.078 | <0.001 |
| Crypt abscess | 31 | 55.4 | 17 | 14.2 | 7.513 | <0.001 |
| Ulcer | 1 | 1.8 | 14 | 11.7 | 0.138 | 0.039 |
| Granuloma | 11 | 19.6 | 7 | 5.8 | 3.946 | 0.005 |
Note: EC: Eosinophil Colitis; N: Number of cases.
Table 2. Results of univariate analysis of cases of eosinophilic colitis compared to non-EC controls.
Table 3 demonstrates the results of multiple logistic regressions. We found that age younger than 6 years, platelet count higher than 250.000, and higher calprotectin level were factors that significantly predict colonic eosinophilia in the pediatric age group. Regression equation, the probability of EC in certain patient, was calculated using the following formula: =-1.22 + 0.659 (Env/food/drug allergy score)+20.236 (weight loss score)+0.491 (ESR score)–0.401 (age score)+2.130 (PLT score)+1.158 (calprotectin score). The accuracy of this equation is 86.4%. Scoring was calculated based on patients’ data as Env/food/drug allergy score (yes/no), weight loss score (yes/no), ESR score (normal/high), age score (numerical), platelet score (normal/high), and calprotectin score (normal/ high).
| Variables | Coefficient | OR | P-value |
|---|---|---|---|
| Env/food/drug allergies | 0.659 | 1.933 | 0.276 |
| Weight loss | 20.236 | 61,44,58,308 | 0.999 |
| ESR | 0.491 | 1.634 | 0.392 |
| Age (<6y and 6y or more) | -0.401 | 0.67 | <0.001 |
| PLT (normal/high) | 2.13 | 8.417 | <0.001 |
| Calprotectin (normal/high) | 1.158 | 3.184 | 0.042 |
Table 3. Results of multiple logistic regressions.
Discussion
Colonic Eosinophilia in children is a major challenge to pediatric gastroenterology since we do not have guidelines for their diagnosis and management. Because the cut-off number of eosinophils in the colonic biopsy is still not estimated, colonoscopy with biopsy remains the only clue for diagnosis. This issue needs a multicenter study to reach a cut-off number that should be used universally among GIT centers.
Although the eosinophilic gastrointestinal disorder is becoming more prevalent in the western world, when it includes the colon, it is still exceedingly rare [14]. Male dominance is highlighted in the literature [18,19]. Correspondingly, boys made up the patients' vast majority in our study. As colonoscopy is still an invasive procedure, we urgently need a non-invasive means to solve this problem. So, we looked to find some predictors for Colonic Eosinophilia to minimize the use of colonoscopy in these children.
Various published studies have discovered varying numbers of diagnostic criteria. Because there is no unanimity on pathologic vs. normal variation in colonic eosinophilia, we did not utilize a number Eos/HPF cut-off to recognize cases. Because clinicians often encounter this situation based on current knowledge, we relied on the biopsies' pathologist interpretation.
In our study, 176 patients with colitis (56 cases and 120 controls) between 2008-2020 were studied in our center, Mansoura city, Egypt. After excluding cases with other colonic diseases such as polyps, infectious colitis, and others, only we looked for cases with colonic eosinophilia according to the pathological report. We found significant factors in the clinicolaboratory data (age, platelet count, and calprotectin level) between controls and individuals with colonic eosinophilia. Until now, it is not easy to compare our results to other studies as the core for differentiation is completely different.
In our study, 43% of cases were found to have no specific diagnosis at the first colonoscopy, so repeated colonoscopies were indicated. In such cases, clinco-laboratory predictors might give us a clue for diagnosis. In our study, the mean age of cases was 3.21, and of controls were 6.49. The younger age correlated more with eosinophilic cases. De Brosse et al. discovered that the patients' mean age with colonic eosinophilia was 10.78 years old [5]. That difference might be due to our large number of cases studied. Nevertheless, the patients' mean age medicated by Behjati et al. was 6.5 years old [20].
Weight loss, diarrhea, and abdominal pain are the colonic eosinophilia clinical manifestations [21]. Nearly all of the patients in our study had diarrhea, with more than 50% having bloody diarrhea and 50% having abdominal pain. Weight loss in children with colonic eosinophilia was not significant in our study. According to de Brosse et al. the most prevalent symptom in patients is abdominal pain [5]. Children's Pensabene's study with colonic eosinophilia found that 75% of them had abdominal pain, nearly 60% had diarrhea, and 50% had bloody stools [22].
While other researchers studied immunological laboratory correlation (IgE, IgA, ASCA, ANCA) 19, we studied the initial platelet count, Erythrocyte Sedimentation Rate (ESR), and fecal calprotectin level at first colonoscopy to find a significant correlation between higher platelet count and high calprotectin level with cases of colonic eosinophilia.
It has been discovered that 50%–75% of cases of colonic eosinophilia coexist with allergic disorders, particularly food allergies to cow's soy, milk protein, and peanuts, and that intervention with an elimination diet is often ineffective [23]. Eosinophils in the mucosa of the large intestine are prevalent in children [22]. Colonic eosinophilia is thought to be an allergic reaction attributed to the prevalence of substantial eosinophilia in the colon, which is linked to a greaer level of total IgE [23]. In our study, total IgE level was not ordered for all our patients.
It is crucial to look for minimally invasive diagnostic techniques because the colonic eosinophilia and IBD clinical picture both emphasize the appearance of non-specific symptoms, particularly at the beginning of the disease. In our study, 30% of cases with EC and 23% of controls were found to be IBD according to the pathology. Therefore, IBD alone is not a good indicator for EC.
As shown in our study, no single factor can differentiate cases from controls; we relied on several factors to answer this issue. We found that enough univariant analysis and multiple regressions could help us at this point. Once again, no single factor is a strong predictor of major etiology, and the holistic clinicopathologic representation must be well-thought-out. Because a large number of cases with colonic eosinophilia do not have the major colonic disease, the diagnostic and therapeutic method must be precision.
Our research has numerous benefits: it is the biggest study of pediatric cases with colonic eosinophilia; it is the only one that compares non-EC controls to colonic eosinophilia; it assesses clinicopathologic form of data with colonic eosinophilia, and it explores the repeat colonoscopy's utility.
Conclusions
The younger the age, higher platelet count, and high fecal calprotectin level at diagnosis of chronic GIT patients can predict the diagnosis of colonic eosinophilia, used as a tool for follow-up response, and impact the whole management plan. The study was limited because it was conducted in a single gastrointestinal referral center and was retrospective in nature. Early detection of colonic eosinophilia might positively impact the patient management plan. In the future, biological markers might help for early diagnosis. There will proceed to be substantial struggle in identifying pathologic colonic eosinophilia until more dependable factors are identified.
What is already known on this topic?
• Colonic Eosinophilia (CE) is a subtype or overlapping with Inflammatory Bowel Diseases (IBD).
• Previous research has not identified clinicopathologic factors that may aid clinicians in distinguishing between these diseases' various groups.
What this study adds?
• Define clinicolaboratory variables that may aid in identifying patients with Colonic Eosinophilia (CE).
• Investigate if such clinicolaboratory predictors could be of value for monitoring the patient's response to treatment.
How this study might affect research, practice or policy?
• Reduce the need for colonoscopes within patients.
• Help clinicians to follow up response non-invasively.
Contributor-ship Statement
• Dr. Mohammad Naghi*: Research design, data refining, clinical data interpretation, writing, revision, and final work approval.
• Prof. Dr. Khaled Zalata: Pathological evaluation of all collected biopsies, contributed to the design, revision, and final approval.
• Dr. Khadega Ali: Contributed in interpretation of pathological data, analysis, and revision.
• Khaleel Wafi: Contributed in interpretation of pathological data, analysis, and revision.
• Prof. Dr. Magdy Ibrahim: contributed with the statistical analysis, and interpretation of data for the work.
• Prof. Dr. Ahmed Abdullah: the Pediatric endoscopist who biopsied the patients, and kept their clinical data, consents. His contributions to the design of the work, critical revision, and final approval.