Current Pediatric Research
International Journal of Pediatrics
Assessment and treatment of pain in pediatric patients.
Halefom Kahsay*
Department of Pharmacy, Collage of Health Science, Adigrat University, Adigrat, Ethiopia.
- Corresponding Author:
- Halefom Kahsay
Department of Pharmacy
Collage of Health Science, Adigrat University
Adigrat, Ethiopia
Tel: +251914257964
E-mail: heleka94@gmail.com
Accepted date: January 30, 2017
Pediatric patients experience pain which is more difficult to assess and treat relatively to adults. Evidence demonstrates that controlling pain in the pediatrics age period is beneficial, improving physiologic, behavioral, and hormonal outcomes. Multiple validated scoring systems exist to assess pain in pediatrics; however, there is no standardized or universal approach for pain management. Healthcare facilities should establish pediatrics pain control program. This review summaries a collection of pain assessment tools and management practices in different facilities. This systematic approach should decrease pediatric pain and poor outcomes as well as improve provider and parent satisfaction.
Keywords
Pain, Pain assessment, Pain management, Pediatric patients
Introduction
According to the International Association for the Study of Pain (IASP) Pain is “an un-pleasant sensory and emotional experience associated with actual and potential tissue damage”. Pain has also been defined as “existing whenever they say it does rather than whatever the experiencing person says” [1-4]. It is one of the most dreading and devastating symptom commonly propagated in peoples with advanced chronic conditions including cancer patents. Pediatric patients are the most under treated and present to hospital for pain compared to adults; because of the wrong belief that they neither suffer pain nor they remember painful experiences [5]. The quality of life experienced by the patient can greatly reduce, regardless of their basic diagnosis. Thus, if pain will be poorly managed, it can reflect the influence on family and careers causing different which may leads to increased rates of hospital admission [5,6]. Uncontrolled pain has also direct impact on health outcomes and more than a few effects on all areas of life. The emotional, cognitive, and behavioral components of pediatric patient are also important to assess pain and to simplify the management practices [7,8].
A long-term negative effect of untreated pain on pain sensitivity, immune functioning, neurophysiology, attitudes, and health care behavior are supported with numerous evidences. Health care professionals’ who care for children are mainly responsible for abolishing or assuaging pain and suffering when possible [5,7,9]. The practice of pediatric pain treatment protocol has made great progress in the last decade with the development and validation of pain valuation tools specific to pediatric patients. Almost all the major children hospitals now have dedicated pain services to provide evaluation and immediate treatment of pain in any child [10,11].
In pediatric age, it is more difficult to assess and treat pain effectively relatively to adults. The lack of ability to notice pain, immaturity of remembering painful experiences and other reasons are the reflection of persistence of myths related to the infant’s ability to perceive pain [12]. However, the treatment of pain in childhood is like the adult management practice which includes pharmacological and non-pharmacological interventions. On the other hand, it critically depends on an in-depth understand of the developmental and environmental factors that influence nociceptive processing, pain perception and the response to treatment during maturation from infancy to adolescence [13,14].
The practice of assessing pain and its management in pediatric patients can show a discrepancy based on the different countries and their respective health institutions. So, this review focused on the contemporary practice and new advances in pediatric pain assessment and its management.
Classification of Pain
Many classification systems are used to describe the different types of pain. The most common classification schemes refer to pain as acute or chronic; malignant or nonmalignant; and nociceptive or neuropathic [15]. Most studies are agreed with the following classification of pain (Table 1).
| Category | Sub-classification | Description |
|---|---|---|
| Pathophysiological | Nociceptive pain | This type of pain arises as the tissue injury activates specific pain receptors named nociceptors, which are sensitive to noxious stimuli. These receptors’ can respond to different stimulus and chemical substances released from tissues in response to oxygen deprivation, tissue disruption or inflammation. It can be somaticor visceralpain based on the site of the activated receptors. |
| Neuropathic pain | This type of pain arises when the abnormal processing of sensory input recognized by the peripheral or central nervous system. | |
| Etiologically | Non-malignant | It includes the pain due to chronic musculoskeletal pains, neuropathic pains, visceral pain (like distension of hollow viscera and colic pain) and chronic pain in some specific anemia. Rehabilitation care is there main treatment protocol. |
| Malignant | This is the pain in potentially life-limiting diseases such as multiple sclerosis cancer, HIV/AIDS, end stage organ failure, amyotrophic lateral sclerosis, advanced chronic obstructive pulmonary disease, Parkinsonism and advanced congestive heart failure. These illnesses are indicating for similar pain treatment that emphases more on symptom control than function. | |
| Based on duration | Acute | This is pain of recent onset and probable limited duration. It usually has an identifiable temporal and causal relationship to injury or disease. Most acute pain resolves as the body heals after injury. |
| Chronic | It is the pain which lasts a long time mostly 6 months, which commonly persisting beyond the time of curing of an injury and may be without any clearly identifiable cause. | |
| Based on location | When Pain is often classified by body site (e.g. on head, on the back or neck) or it can be the anatomic function of the affected tissue (e.g. vascular, rheumatic, myofascial, skeletal, and neurological). It does not provide a background to resolve pain, but it can be useful for differential diagnoses. | |
Table 1: The general classification of pain in pediatrics [3,4,8,15-20]
Assessment of Pain in Pediatrics
Pain is often referred to as the “fifth vital sign” and it should be assessed and recorded as often as other vital signs. The appropriate intervention of pain is planned based on the accurate valuation of pain. Organized and routine pain assessment by using the standardized and validated measures is accepted as a corner stone for effective pain management in patients, unrelatedly to the age or other conditions [21]. A study in Brazil suggests that consistent accomplishment of assessments of pain using ordinary scales, such as Face, Legs, Activity, Cry and Consolability score and other bodily parameters are mandatory to optimize pain management in pediatric intensive care units [22]. As pain is a subjective experience, individual self-reporting is the favorite method for assessing pain. However, when valid self-report is not available as in children who cannot communicate due to age or developmental status, the observational and behavioral assessment tools are acceptable substitutions [5,7,22].
The use of the pain management algorism on Stollery children’s hospital shows significant improvement for assessment of pain in pediatrics. The pre and post analysis indicated in a staff (n=17) given that a feedback of 41.2% felt that the algorism improved their ability to assess and manage pain in children equally, 35% felt that it increased their capacity to communicate a child’s pain with other health care team members, 52.9% felt that the algorism should be further applied on other units across the hospital [23]. Even though, the assessment of pain symptoms is easy in adults, selection of appropriate pain assessment tools should consider age, cognitive level and the presence of eventual disability, type of pain and the situation in which pain is occurring in children. Therefore, healthcare professionals need to be aware of their limitations in addition to trained in the use of pain assessment tools [7,24,25].
The assessment in Canadian pediatric teaching hospitals indicated out of 265 children, majority (63%) of them found with a minimum of one documented pain assessment tool, 30% of children had at least two assessment tools, 17% had 3-5 measurement tools and 16% had at least six assessments in 24 h of admission. Most (63%) of the children were find a different document of 666 pain assessment tools, with a median of three assessments per one child [14]. Parent, patient, as well as staff satisfaction is positively associated with accurate assessment of pain in addition to well improvement of pain management. Brief and well validated tools are available for the assessment of pain in non-specialist settings. Nevertheless, each tool cannot be broadly suggested for assessment of pain in all children and across all settings. Individual needs of the children lead to assess and re-evaluate of pain consistently as a mandatory in every situation. On top of that, ethnicity, language, and cultural factors should be under consideration as they may influence pain assessments and its expression [5,12,26].
Most formal and commonly used means of pediatric assessment tools for pain are available and categorized depending the pediatrics age.
Pain Assessment in Neonates
Neonates pain rating scale (NPR-S): Major guidelines indicate that the assessment of pain in neonates (term babies up to 4 weeks of age) had better be use the Crying, Requires oxygen for saturation above 95%, Increasing vital signs, Expression and Sleepless (CRIES) scale (Table 2) [2,24,27-30].
| Cries Pain Rating Scale | |||
|---|---|---|---|
| 0 | 1 | 2 | |
| Crying | No | high pitched | inconsolable |
| Requires O2 for sat >95% | No | <30% | >30% |
| Increased vital signs | HR and BP <or=pre-op | HR and BP; Increased <20% of pre-op | HR and BP; Increased >20% of pre-op |
| Expression | None | Grimace | Grimace/grunt |
| Sleepless | No | Wakes at frequent intervals | Constantly awake |
Table 2: Neonatal pain rating scale [27-29]
Several other pain scales have been designed for the objective assessment of neonatal pain, including the COMFORT (“behavior”) score, pain assessment tool, scale for use in newborns, distress scale for ventilated newborns and infants. Although these assessments are validated as research tools, the mainstay of appropriate management includes the caregiver’s awareness, knowledge of clinical situations where in pain occurs, and sensitivity to the necessity of preventing and controlling pain [31].
Assessment of pain in infants: On a study in Australia hospitals, Infants (1 month to approximately 4 years) were scored using the face, leg, activity, cancelability and cry (FLACC) measuring tool. Scoring should be done by staff after observing the infant for 1 min. Among two observers a reliability of FLACC was established in a total of 30 children in the post anesthetics care unit (PACU) (r=0.94). After analgesic administration, validity was established by demonstrating a proper decrease in FLACC scores. Correspondingly, a high degree of association was found between PACU nurse’s global pain rating scale, FLACC scores, and with the objective scores of pains scale. This tool has been established in various settings and in diverse patient populations and finds that as reliable and valuable. It provides a simple background for computing pain behaviors in children who may not be able to put into words the incidence or severity of pain. Lastly, the constructed validity is supported by analgesic administration as the scores decreases significantly. Another recent studies have demonstrated that FLACC was the most chosen in terms of sensible qualities by clinicians at their respective institutions [27,29,32-35]. Although the tool can be used by clinicians, it is more effective with parent input to provide a description of ‘baseline’ behavior. This is supported by the findings of the Malvinas study, which suggested that the addition of unique descriptors allowed parents to augment the tool with individual behaviors unique to their children. In addition, for infants who show good comprehension and motor skills, this pain assessment tool can be used as an alternative [36]. The FLACC scale has 98% sensitivity and 88% specificity in assessing pain levels [34]. Therefore, those different studies concluded that FLACC scale is the most appropriate measurement tool for pain assessment in infants (Table 3).
| FLACC Behavioral Pain Assessment Tool | ||||
|---|---|---|---|---|
| 0 | 1 | 2 | ||
| Face | No particular expression or smile | Occasional grimace/frown withdrawn or disinterested | Frequent/constant quivering chin, clenched jaw | |
| Legs | Normal position or relaxed | Uneasy, restless or tense | Kicking or legs drawn up | |
| Activity | Lying quietly, normal position, moves easily | squirming, shifting back and forth, tense | Arched, rigid or jerking | |
| Cry | No cry | Moans or Whimpers, occasional complaint | Crying steadily, screams or sobs, frequent complaints | |
| Cancelability | Content or relaxed | Reassured by occasional touching, hugging or being talked to, distractible | Difficult to console or comfort | |
Table 3: FLACC assessment tool [27,29,32-35]
Assessment of pain in older children:
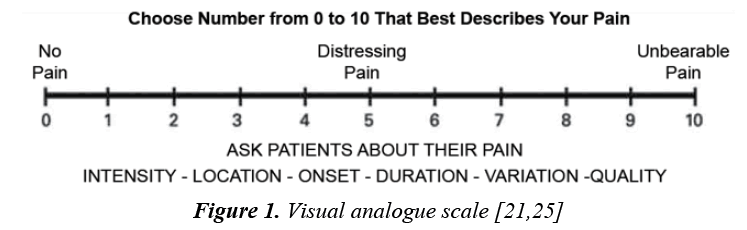
Self-report: The single most reliable indicator of the existence and intensity of pain and any resultant distress is the patient’s self -report. For older children, the use of a self-reporting scale can be helpful to staff and empowering to the patient [24]. A self-report of pain from a patient with limited verbal and cognitive skills may be a simple yes/no or other vocalizations or gestures, such as hand grasp or eye blink. When self-report is absent or limited, explain why selfreport cannot be used and further investigation and observation are needed [4,28]. Myriad guidelines are coming together in using different self-report methods in assessing pain in older children such as the Visual Analogue Scale (VAS) (Figure 1) which is described by a horizontal line with “no pain” at the beginning to “worst possible pain” at the termination and patients draw a line to show their severity of pain. It has several benefits: it avoids imprecise descriptive terms, quick and simple to score, and offers many determining points. However, it can be difficult in post-operatively or in children with neural and psychological disorder as it needs a concentration and coordination [1,12,24,29]. Wong-Baker faces pain rating scale is the other selfreport tool mainly used to assess acute pain. Expressed by six line-drawn faces range from no pain at one end to worst pain at the other end and assigns by number with word descriptors to each face to indicate the intensity of pain [11,37].
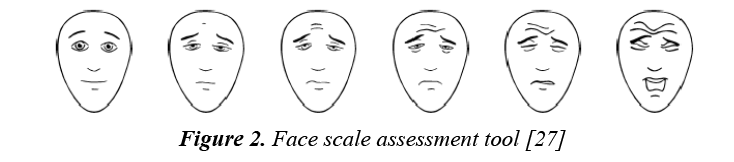
However, many studies take a type of self-report, face scale method for assessing pain in older children. Below is the Faces scale, currently used by the children’s hospital at West Mead:
These faces show how much something can hurt. From no pain to very much pain pointing to the face by the patient him/herself to show how much he/she hurt to simplify pain assessment (Figure 2).
Generally, most institutions approved using the pain assessment tools as their basic instrument for diagnosis and management of the different type of pains encountered in pediatrics.
Management of Pain in Pediatrics
The management of pain in pediatrics is still misunderstood. Explicitly, neonates and infants are not managed for pain effectively, due to the misperception that they are not able to sense pain as adults [16,18]. American academy of pediatrics suggested that the lack of pain assessment and fears of adverse effects of analgesic medications including respiratory depression and addiction are the main barriers to the treatment of pain in children [9]. As the underlying disease is expected to advance a continuous adjustment of pain therapy is required. A study in Toronto hospitals shows that out of the total 265 children, 58.9% received a minimum of one documented intervention of pain management. Out of 66 children with recognized pain (mild, moderate or severe), 55 of them received an intervention for their pain [14]. It extends beyond pain relief, encompassing the patient’s quality of life and ability to work productively and to enjoy recreation. Pain management is a joint responsibility among the members of the health care team. This includes addressing pain status of each patient daily on inpatient unit rounds or with each patient visit, consultation if pain treatment is ineffective, and discharge planning for continuing pain management needs [3,15,38].
Generally, on consideration of the above challenges Managements of pain in pediatrics encompass the use of pharmacological and non-pharmacological interventions to control the patient’s identified pain.
Non-Pharmacological Interventions
Non-pharmacological measures should be favored as base line for both adults and children intervention of pain. in conjunction with pharmacological options to help lower levels of anxiety, pain and distress, the psychological comfort measures such as relaxation techniques and distraction as well as physical interventions including the use of massage repositioning or heat and/or cold compresses are useful Strategies [3,4,15,19,24,39]. According to the guidelines for clinical practice of the American pain society, pain education such as the interventions and options for pain relief during the pre-surgical visit for patients and their families is important to develop their perception towards pain management [40]. A study by Lm Zhu et al. in Canadian pediatric teaching hospitals indicated that out of the 55 (83.3%) children who take pain management intervention, six of them received a physical treatment and five children received a psychological intervention [14].
General the following interventions are considered as nonpharmacological treatment of pain based on the recent and numerous studies.
Sucrose
Concentrated sucrose solutions (2 ml of 24% solution) may be used as a pain relief measure in preterm and term newborns up to 1 month of age as its analgesic effect lasts approximately 3 to 5 min. It promotes natural pain relief by activating endogenous opioids in contact with the oral mucosa. The effectiveness of sucrose solution enhanced by allowing the infant to continue sucking on a pacifier or breastfeed [41]. A randomized controlled clinical trial found that a single dose oral sucrose is effective and safe for minimizing physiological response to a painful stimulus and behavioral expressions in preterm infants [37]. The proposed hypothesis initiated from the endogenous opioid release can cause by taking oral 20- 30% glucose through unknown mechanism. Therefore, Several studies recommended to considered oral sucrose as one of the non-pharmacological interventions of pain [30,31,37].
Distraction
Distraction involves engaging a child in a wide variety of pleasant activities that help focus attention on something other than pain and the anxiety. Examples of distraction activities are listening to music, singing a song, blowing bubbles, playing a game, watching television or a video, and focusing on a picture while counting. Guided imagery and breathing techniques may be forms of distraction for school-age children and adolescents [42]. A randomized control trial suggested that a virtual reality games were found to be effective distraction for children with acute burn injuries [43].
Breast Feeding
Breast milk is the best alternative to no intervention or to the use of sucrose in patient suffering with a single painful procedure. During venipunctures and heel stick procedures, neonates who were breastfed showed a substantial decrease in the variability of physiologic response as compared to other non-pharmacological interventions [30,39,44].
Skin-to-Skin Contact
Skin to skin contact demonstrated as effective nonpharmacological intervention in reduction of pain especially when used as adjunctive therapy to breastfeeding or other sweet solutions. Canadian medical association demonstrated that skin-to-skin contact principally Kangaroo care plays its own role in reducing and caring their children as the care giver and the baby have a direct physical contact [4,30].
Pharmacological Management of Pain
The current pharmacologic treatment protocol of pain for children is primarily extrapolated from adult intervention without any evidence of value in children [32]. Highquality pediatric experimental researches are needed to demonstrate efficacy and safety of analgesics for innumerable pain conditions in children to avoid continued use of analgesics empirically [8]. The development of ageappropriate pain assessment tools leads to improvement in the management of pain in children in the last two decades. Depending on the severity of pain, non-opioids and opioids are the most common analgesic agents used a “step-wise” approach in management of pain in both children and adults [19,24,28]. It is important that pain be reassessed soon after any pharmacological intervention to guide further interventions and to ensure the achievement of pain relief ensured by reassessment of pain regularly after any pharmacological intervention. Multimodal analgesia practice should be considered in patients with pain by concomitant use of the opioids, NSAIDs and other adjuvant therapies [14].
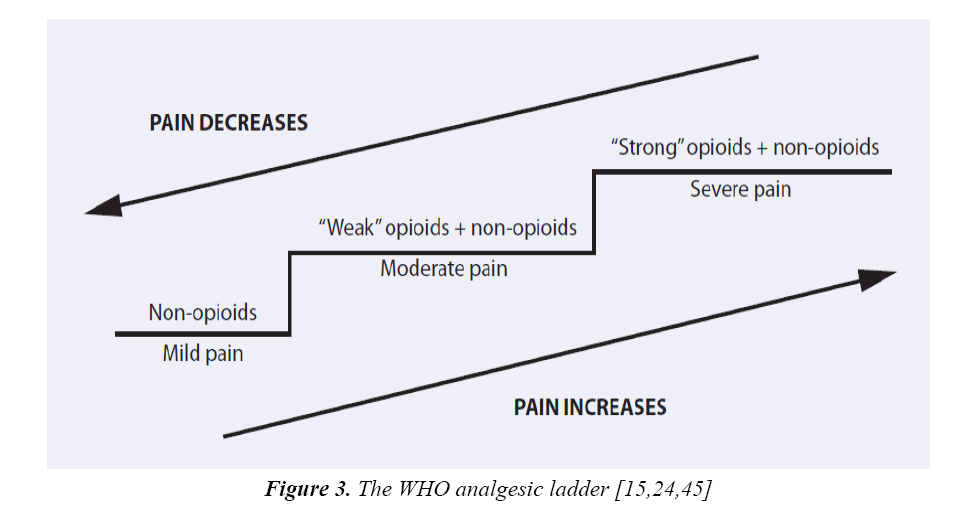
Generally, World Health Organization (WHO) demonstrated three-step analgesic ladder for treatment of pain (Figure 3) [45].
Non-Opioids Used for Management of Pain in Pediatrics
Acetaminophen: It is the most frequently used painrelieving agent in pediatric patients. It has lack of significant side effects and excellent safety profile with benefit to all levels of pain in children [39]. In common to the guideline of different institutions (Table 4), initially a loading dose of 30 mg/kg should be given, then 10-15 mg/ kg every four to six hours as maintenance with maximum dose of 90 mg/kg/day for children. But, for term neonates of less than ten days 60 mg/kg and 45 mg/kg for premature infants. Neonates have a slower clearance rate so the drug must be given less frequently. Acetaminophen is manly used for mild to moderate pain independently and in combination of opioids for patients with severe pain for example acetaminophen with codeine) [24,37,45]. Rectal preparations of this analgesics used for infants and toddlers who are unable or unwilling to take orally. However, several studies have confirmed that rectal absorption comparatively inefficient and slow. Hepatotoxicity is not associated with single rectal doses of 30 to 45 mg/kg produced plasma concentrations that were generally in the effective range [46].
| Drug | Oral peak time | Usual Pediatric dosage | Usual Adult dosage | Comments |
|---|---|---|---|---|
| Acetaminophen | 0.5–2 h | 10–15 mg/kg every 4 h orally 20-40 mg every 6 h rectally | 650–1000 mg every 4 h | Lacks the peripheral anti-inflammatory activity of other NSAIDs |
| Choline magnesium trisalicylate (Trilisate) | 2 h | 25 mg/kg every 12 h | 1000–1500 mg every 12 h | Does not increase bleeding time like other NSAIDs; available as oral liquid |
| Ibuprofen | 0.5 h | 6–10 mg/kg every 6–8 h | 200–400 mg every 4–6 h | Fewer GI effects than other non-selective NSAIDs |
| Naproxen | 2–4 h | 5 mg/kg every 12 h | 250–500 mg every 6–8 h | Delayed-release tablets are not recommended for initial treatment of acute pain |
| Ketorolac | 0.75–1 h | 0.25–0.5 mg/kg IV or IM, every 6 h | 30 mg IV loading dose, then 15–30 mg every 6 h | IV or IM use only in children less than 50 kg; should not be used for children with bleeding disorder or at risk for bleeding complications |
| Celecoxib | 3-6 h | 1-2 mg/kg | 100-200 mg every 12 h | sparing of COX-1 reduces the risk of serious GI side effects and renal toxicity Also, no effects on platelet aggregation |
Table 4: Dosage guidelines for the common non-opioids used in the management of pain in pediatrics [12,48]
In relative to oral doses rectal doses are slowly decline in plasma concentrations. Based on a day pharmacokinetic study, the dosing interval for rectal dose extended to at least 6 h [29]. Acetaminophen toxicity can result when the toxic metabolite acetyl-p-benzoquinone-imine (NAPQI) is produced in high quantities. This may lead infants and children to hepatotoxicity. However, rodent study compared weanling to adult rats and suggested that infants produce high levels of sulfhydryl group of glutathione (GSH) to bind NAPQI as a part of hepatic growth and this may provide some protection against the hepatotoxicity produced by overdose [7].
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
NSAIDs are commonly used analgesics with less contraindication in relative to opioids. Mainly these are used as analgesic regimen in mild and moderate pain by preventing the conversion of arachidonic acid to prostaglandins and thromboxane. Prostaglandins are pro inflammatory mediators that sensitize nociceptors to increase afferent nociceptive signal to pain. Diclofenac, ketoprofen and ibuprofen commonly used NSAIDs in pediatric practice [7]. An observational study on the use of non-steroidal anti-inflammatory drugs (NSAIDs) was done in a sample of 51 patients in Italy resulted that ibuprofen was the most (68.6%) used NSAID followed by ketoprofen 9.8% and acetylsalicylic acid 7.8% for pain management of in pediatrics. The use of NSAIDs is now well established in clinical pain management [47].
This show to decrease morphine consumption and improve the quality of analgesia without increasing the incidence of side effects. These drugs are now a standard peri-operative analgesic agent in many pediatric institutions. Ibuprofen mainly used is available in oral suspension, infant drops, tablet and intravenous formulations. It is used to close patent ductus arteriosus (PDA) and as pain reliever in perioperative in neonates and children weighing greater than 7 kg. It is available as different dosage form such as oral suspensions, tablets, infant drops and intravenous preparations with a dose of 30 mg/kg in 3-4 divided doses. Besides, diclofenac is available like ibuprofen dosage forms with the recommended dose to children at a dose of 0.3–1 mg/kg with a maximum dose of 50 mg 3 times daily. However, ketorolac is not approved for use in children under 16 years of age. It only used for short term interventions of acute post-operative pain at a dose of 10- 40 mg every 4-6 h for a maximum of 7 days [7,12,48].
Meta-analysis of studies comparing ibuprofen and diclofenac reveal that both drugs work well and that choosing between them is an issue of dose, safety and cost. An oral ibuprofen dose of 30-40 mg/kg per day appears to render equivalent analgesia to oral/rectal diclofenac 2-3 mg/kg per day. No difference in safety has been documented in these dose ranges [27]. Clinical pharmacology understanding for non-opioid analgesics is required for optimal administration. Because, for patients with post-operative pain, the minimal effective for analgesic dose and toxic dose is not known certainly. These doses may be higher or lower than the usual dose ranges recommended for the drug involved. On top of that, NSAIDs and acetylsalicylic acid have a potential toxicity, most commonly bleeding diathesis due to inhibition of platelet aggregation, renal impairment and gastro duodenopathy due to prostaglandin inhibition [12].
Opioids
Like adult population, management of acute pain in pediatric is also targeted with opioids. The analgesic effect comes through binding the mu-opioid receptor which is widely distributed at sites of peripheral inflammation and throughout the CNS. The variation in pharmacological response of opioids in pediatrics leads to adjustment based on clinical response, age and presence of side effects [7,27]. The indications for opioids include postoperative pain, pain due to sickle cell disease, and pain due to cancer [49]. A study in the Canadian teaching hospitals confirms that opioids are mainly used in severe pain and shows an improvement in all patients from the their experience of severe pain received an opioid treatment [14]. Most currently practiced guidelines in recent advanced pediatric hospitals are commonly used the following opioids in the management moderate to severe pain in pediatrics (Tables 5-7).
| Age Group | Volume of Distribution (l/kg) | Clearance (ml/kg/min) | Half Life (Hours) |
|---|---|---|---|
| Preterm neonate | 1.8-5.2 | 2.7-9.6 | 7.4-10.6 |
| Term neonate | 2.9-3.4 | 2.3-20 | 6.7-13.9 |
| 1-8 Years | 1.4-3.1 | 6.2-56.2 | 0.8-1.2 |
| Adult | 1.1-2.1 | Dec 34 | 1.4-3 |
Table 5: Pharmacokinetics of morphine [27]
| Age | Appropriate Initial Dose |
|---|---|
| 1-6 Month | 50-150 µg/Kg every 4 h |
| 6 Month-12 years | 100-300 µg/Kg every 4 h |
| 12-18 years | 3-20 mg every 4 h |
Table 6: Dose administration of morphine [7,12]
| Drugs | Usual Recommended Starting Dose | Comments | |
|---|---|---|---|
| Oral | Parenteral | ||
| Morphine | 0.3 mg/kg every 3–4 h | 0.1 mg/kg every 3–4 h | Used as a standard of comparison for all opioid drugs |
| Codeine | 0.5–1 mg every 3–4 h | Not recommended | Codeine is a pro-drug and not all patients convert it to an active form to achieve analgesia |
| Oxycodone | 0.1–0.2 mg/kg every 3–4 h | Not recommended | Use as first line therapy for severe pain |
| Methadone | 0.2 mg/kg every 4–8 h | 0.1 mg/kg every 4–8 h | 0.1mg/kg commonly used for acute pain 0.2-0.4 mg/kg commonly used for chronic pain |
| Fentanyl | 5–15 mcg/kg Oralet | 1 mcg/kg every 1–2 h | The Oralet is not widely used because of nausea and vomiting side effects |
Table 7: Opioids commonly used in pediatric pain management [12,50,51]
Morphine: It is the most commonly used phenanthrene derivative opioid in children with severe pain. Pharmacokinetics disparity (Table 5) exists for this drug between age groups. Because the plasma concentrations of morphine in neonates and infants display a prolonged half-lives (2-3fold) difference even with administration of constant infusion [7,12,27].
Codeine: It is a prodrug which activated to morphine by the enzyme cytochrome CYP2D6. However, the activity of this enzyme is highly variable and shows inter-individual variation which leads to a variation in analgesic effect of codeine [7,10]. Caucasian population are considered as ‘Super Metabolizers’ whose approximately carry 10% of this variant. Therefore, even low dose codeine put them at risk of respiratory depression and excess sedation. Indeed, codeine is now infrequently prescribed in Australia [7,27].
Tramadol: It is structurally related to morphine which has a central analgesic effect by the formation of O-desmethyl-tramadol with a mu-opioid receptor affinity 200 times greater due to biotransformation in the liver by cytochrome P450(10). A dose of 50–100 mg every 4 h to a maximum of 400 mg per day is recommended to children between 12–18 years [7]. However, now a day’s tramadol does not recommend for pediatrics under 12 years of age.
Fentanyl: Even though it is metabolized to inactive metabolites, fentanyl has 100 times more effect of analgesic than morphine. Commonly it used by the trans mucosal, intravenous, inhalational or intra-nasal and transdermal routes for procedural related pains in surgery due to its rapid onset and offset [7]. Case series and outcome studies of children not undergoing intubation suggest a higher frequency of opioid-induced respiratory depression among neonates than among infants over six months of age or older children [27]. In addition to the use of naloxone 10-20 mcg /kg for urgent situations, deep breath encouragement, awakening of the patient and withholding further doses may manage mild respiratory depression in children. Non-respiratory side effects of opioids, including nausea, ileus, itching, and urinary retention, are common among infants and children and may cause considerable distress. Many opioid side effects can be ameliorated by drug therapy directed at the side effect (e.g. antiemetic’s to treat nausea and vomiting, antihistamines to treat itching and laxatives to treat constipation) [12,49].
Generally, WHO guideline recommends analgesic treatment in two steps according to the child’s level of pain severity [15,24,48].
Indication Based on Pain Intensity
• Stage 1 – Non-opioid +/- adjuvant agent for mild pain
• Stage 2 – Opioid +/- non-opioid +/- adjuvant agent. For moderate to severe pain or pain uncontrolled after Step 1.
Common Analgesic Adjuvant
When a drug has a primary indication other than pain but is analgesic in some conditions it can be describe as adjuvant analgesic. Such adjuvants mainly used for the treatment of non-malignant pain in combined with primary analgesics to improve the outcome and to maintain the balance between relief and side effect [12]. Moreover, adjuvants can provide independent analgesic activity and treat concurrent symptoms that exacerbate pain for specific types of pain. The most commonly used adjuvants such as anti-depressants (amitriptyline), topical and local anesthetics and anticonvulsants (e.g. gabapentin and pregabaline) for neuropathic pain, steroids in edema induced pains, bisphosphonates and radiation therapy for metastases bone pain, neuroleptics for pain associated with anxiety, restlessness or nausea) [7,27,52].
Conclusion
In summary, numerous clinical practice guidelines and policy statements have been published in the last 10 years about pediatric pain. These publications are valuable resources for physical therapists and other health care providers who serve infants, children, and adolescents who have, or are at risk for, pain resulting from diverse etiologies. Improved management is contingent on valid and reliable measurement of pain. Fortunately, there are many excellent pediatric pain measures. Selection of appropriate measures requires an understanding of pain, measurement, and child development. Because, measurement of pain in infants, young children, and children with disabilities who are unable to selfreport is particularly challenging and merits increased attention. These assessment tools have a basic benefit to the health care providers who are involved in pediatric health management to control the pain through nonpharmacological and pharmacological interventions. On top of that, pediatric institutions are well positioned to support and implement policy initiatives to improve the identification and management of pediatric pain and to contribute new knowledge through research.
Recommendations
An appropriate pain assessment measurements and techniques are needed to manage pain in pediatric patients and should be applied in every pediatric health care institution. Firstly, high possible standard of pain care for all patients should be provided through a multi modal (non-pharmacological, pharmacological and adjuvants) approach. Secondly, pediatric centers collaboration will be necessary to share the standard treatment protocol. Finally, even though the incidence of pain in children is like that of adults, clinicians should have considered the distinctiveness of children. The Cooperation of the caregivers and families are essential for successful pain assessment and its intervention in pediatric patients.
References
- O’Rourke D. The measurement of pain in infants, children and adolescents: from policy to practice. Physical Therapy 2004; 84: 560-570.
- Harris J, Ramelet AS, Dijk Mv, et al. Clinical recommendations for pain, sedation, withdrawal and delirium assessment in critically ill infants and children. Intensive Care Med 2016; 42: 972-986.
- Hospital TJH. Interdisciplinary clinical practice manual. Pain, Assesment and management 2001.
- Gerik SM. Pain management in children: Developmental considerations and mind-body therapies. South Med J 2005; 98: 295-301.
- General Palliative Care Guidelines for the Management of Pain at the End of Life in Adult Patients 2011.
- Walters MA. Pediatric pain letter, pain assessment in Sub-Saharan Africa. International Association for the Study of Pain 2009; 11: 1.
- Nair S. Pediatrics pain: Physiology, assessment and pharmacology England: Cardiff University Hospital 2013: 289.
- Assessment and management of children with chronic pain. American Pain Society 2012.
- Joseph FHJ, Coleman WL, Foy JM. The assessment and management of acute pain in infants, children and adolescents. Pediatrics 2001; 108: 1.
- Verghese ST, Hannallah RS. Acute pain management in children. J Pain Res 2010; 3: 105–123.
- Canbulat N, Kurt AS. Pain management and nursing approaches in pediatric oncology. 2012.
- Chiaretti A, Pierri F, Valentini P. Current practice and recent advances in pediatric pain management. Eur Rev Med Pharmacol Sci 2013; 17: 112-126.
- Guidance on competencies for paediatric pain medicine. In: Ansthetistes, editor. Great Britain and Ireland 2010.
- Zhu LM, Stinson J, Palozzi L, et al. Improvements in pain outcomes in a Canadian pediatric teaching hospital following implementation of a multifaceted, knowledge translation initiative. Pain Res Manage 2012; 17: 173-179.
- Mcpherson ML, Canaday BrR, Heit HA, et al. A pharmacist’s guide to the clinical assessment and management of pain. In: Science PPa, editor. University of Maryland, Baltimore: American Pharmacist Association. 2004.
- Pain Management Guidelines California: Medical Board of California. 2014.
- Cole BE. PAIN management: Classifying, understanding and treating pain. 2002: 23.
- Kumar N. WHO normative guidelines on pain management. Geneva. 2007.
- Pain management across the life span: From pediatrics to geriatrics. In: Center SM, editor. Sacramento. 2009.
- Manchikanti L, Falco FJE, Singh V. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part I: Introduction and general considerations. Pain Physician Journal 2013; 16: 1533-3159.
- Infants and children: A guideline on management of acute and procedural pain in the emergency department space. North Sydney W: NSW, Minister of Health 2016.
- Dantas L, Dantas T, Santana-Filho V, et al. Pain assessment during blood collection from sedated and mechanically ventilated children. Rev Bras Ter Intensiva 2016; 28: 49-54.
- Reid K, Lukenchuk L, Shannon, et al. Does a pain algorithm improve pain assessment and management? Pain aligorism. Stollery Childrens Hospital. 2012.
- Wong C, Lau E, Palozzi L, et al. Pain management in children. Pharm J 2012; 145: 222-225.
- Solodiuk J, Curley MAQ. Pain assessment in non-verbal children with severe cognitive impairments: The individualized numeric rating scale (INRS). J Pediatr Nurs 2003; 18.
- Square G, Crescent SG. Management of chronic pain. Scotland: Scottish Intercollegiate Guidelines Network. 2013.
- Pain management at CHW practice guideline. In: Department A, editor. Children’s hospital at West Mead: Staff Anaesthetist. 2015.
- Herr K, Coyne PJ, McCaffery M, et al. Pain assessment in the patient unable to self-report: Position statement with clinical practice recommendations. Pain Manag Nurs 2011; 12: 230-250.
- Evans M. Pediatric pain management. In: Center PM, editor. Chicago American Medical Association. 2010.
- Witt N, Coynor S, Edwards C, et al. A guide to pain assessment and management in the neonate. Curr Emerg Hosp Med Rep 2016; 4: 1-10.
- Khurana S, Hall RW, Anand KJS. Treatment of pain and stress in the neonate. Neurology 2005; 6.
- Linhares MBM, Oliveira NCAC, Doca FNP, et al. Assessment and management of pediatric pain based on the opinions of health professionals. Psychology and Neuroscience 2014; 1: 43 - 53.
- Malviya S. Assessment of pain in children. In: Ann Arbor M, editor; University of Michigan. 2006.
- Ramira ML, Instone S, Clark MJ. Pediatric pain management: An evidence-based approach. Pediatr Nurs 2016; 42: 39-46.
- Mandee S, Suraseranivongse S, Teerachanant T, et al. Educational video to improve the knowledge of health care providers in pain assessment of preschool children. Siriraj Med J 2012; 64: 22-26.
- The recognition and assessment of acute pain in children clinical practice guidelines. Update of full guideline. 20 Cavendish Square, London: Published by the Royal College of Nursing. 2009.
- Atkinson P, Chesters A, Heinz P. Pain management and sedation for children in the emergency department. Br Med J 2009; 339.
- MacLaren JE, Cohen LL. Teaching behavioral pain management to healthcare professionals: A systematic review of research in training programs. J Pain 2005; 6: 481-492.
- Management of pain in children: Pain guidelines 2006.
- Glowacki D. Effective pain management and improvements in patients’ outcomes and satisfaction. Crit Care Nurse 2015; 35: 33-43.
- Morash D, Fowlder K. An evidence based approach to changing practice: Using sucrose for infant analgesia. J Pediatr Nurs 2004; 19: 366-370.
- Pain assessment and management in children. 2009.
- Das DA, Grimmer KA, Sparnon LA, et al. The efficacy of playing a virtual reality game in modulating pain for children with acute burn injuries: A randomized controlled trial. BMC Pediatr 2005; 5: 1471-2431.
- Taddio A, Shah V, Leung E, et al. Knowledge translation of the HELPinKIDS clinical practice guideline for managing childhood vaccination pain: Usability and knowledge uptake of educational materials directed to new parents. BMC Pediatr 2013; 13.
- WHO guidelines on the pharmacological treatment of persisting pain in children with medical illnesses. Geneva, Switzerland. 2014.
- Yung A, Thung A, Tobias JD. Acetaminophen for analgesia following pyloromyotomy: Does the route of administration make a difference? J Pain Res 2016; 9: 123-127.
- Cardile S, Martinelli M, Barabino A, et al. Italian survey on non-steroidal anti-inflammatory drugs and gastrointestinal bleeding in children. World J Gastroenterol 2016; 22: 1877-1883.
- Hauer J, Duncan J, Scullion BF. Pediatric pain and symptom management guidelines. In: Team PAC, editor. Boston Children’s Hospital Dana farber Cancer Institute. 2014.
- Berde CB, Ethna NFS. Analgestics for the treatment of pain in children. N Engl J Med 2002; 347.
- Pain: Current understanding of assessment, management and treatments. 2001.
- http://www.prenhall.com/london
- Policies and procedures: pain management – Pediatric acute care. In: Nursing, editor. Royal University Hospital, SHR Interprofessional Practice. Pediatric Pain Management Committee. 2012.