Current Pediatric Research
International Journal of Pediatrics
Accidental intravenous administration of enteral formula through a central line in an infant.
Sulafa Sindi, Morouj Kandil, Mohammed Ghazi*
Department of Pediatrics, King Abdulaziz University Hospital, Jeddah, Saudi Arabia
- Corresponding Author:
- Mohammed Ghazi
Department of Pediatrics,
Faculty of Medicine,
King Abdulaziz University,
Jeddah, Saudi Arabia
E-mail: Mgaze@kau.edu.sa
Received: 24 January, 2023, Manuscript No. AAJCP-23-88620; Editor assigned: 26 January, 2023, Pre QC No. AAJCP-23-88620(PQ); Reviewed: 10 February, 2023, QC No. AAJCP-23-88620; Revised: 17 February, 2023, Manuscript No. AAJCP-23-88620(R); Published: 27 February, 2023, DOI:10.35841/0971-9032.27.02.1802-1805.
Medication error is common in hospitalized patients. However, milk ingestion through an intravenous line is a rare entity that has fatal medical sequel if managed late or misdiagnosed.
A rare case report of an 11 month old infant who received a 5 ml of intravenous formula milk through central line with subsequent complications of sepsis and acute respiratory distress syndrome within few minutes of ingestion. Moreover, complications of acute renal failure along with hepatic insufficiency and coagulation disorders could happen.
These errors are possible due to compatible connections between intravascular catheter and enteral feeding catheter.
Confusion between lines connected to the patient could occur, either due to system or human errors. Healthcare system has a major role in elaborating the use of each line connected to the patient whether to the patient’s family or entitled caregiver to decrease the potential morbidity or mortality of children.
Keywords
Pediatrics, Central line, Enteral feed.
Introduction
Medication errors are widespread and can present as errors in prescription, preparation, or administration [1]. They contribute to up to 4% of all inpatient hospitalizations [2]. Accidental milk injection errors through the venous line were common 42 years ago and have continued to challenge the healthcare system today [3].
Accidental administration of enteral nutrition intravenously may result in unfavorable, even fatal, consequences including sepsis and multi-organ failure [4,5]. Misconnections due to multiple tube types, such as peripheral and central lines, feeding tubes, epidural catheters, and peritoneal catheters, can lead to several complications [6-8].
Here in, we present a unique case of an 11-month-old boy who received a 5 ml of formula milk through a central intravenous catheter, as well as the complications and further management with the aim to prevent similar events in the future.
Case Presentation
An 11-month old boy with a known history of tracheoesophageal fistula complicated by esophageal stenosis after button battery ingestion was admitted to the pediatric ward by the gastroenterology team for esophageal dilation using upper endoscopy.
The patient was connected to a cardiopulmonary monitor while having a Nasogastric Tube (NGT) for feeding and a left central line in place for blood sampling and medication administration. His caregiver observed the bedside nurse administering formula by a syringe through the NGT and was shown a demonstration of this procedure.
However, the caregiver later administered 5 ml of formula milk intravenously through the central line. They instantly recognized the error and informed the bedside nurse. Upon examination, the patient was tachypneic (respiratory rate of 75/ min) and tachycardic (heart rate of 180 beats/min), with an oxygen saturation of 80%, normal blood pressure, mottled skin, and a Glasgow coma scale score of 9.
The patient was connected to a non-rebreather facemask to improve oxygenation. An Electrocardiogram (ECG) showed normal sinus tachycardia with no arrhythmia.
The pediatric intensivist on-call was contacted. The patient received midazolam and fentanyl for sedation; he was intubated with a cuffed 4 cm endotracheal tube.
The patient was transferred to the Pediatric Intensive Care Unit (PICU) because of the increased risk of fat embolism, Disseminated Intravascular Coagulation (DIC), and multiorgan failure.
Results
A thorough workup was performed, including complete blood count, venous blood gas, Liver Function Tests (LFT), coagulation profile, renal function tests, creatine kinase assessment, lipid profile assessment, troponin-I assessment, and blood culture from the central and peripheral lines (Table 1).
| Test Name | Result | Unit | Reference range |
|---|---|---|---|
| Complete blood count | |||
| White Blood Cell (WBC) | 5.55 | × 109/L | 6-18 |
| Hemoglobin (Hb) | 10.1 | g/dL | 10.4-15.6 |
| Hematocrit (HCT) | 33 | % | 35-51 |
| Mean Cell Volume (MCV) | 73 | fL | 78-102 |
| Mean Cell Hemoglobin (MCH) | 22.3 | pg | 32-36 |
| Platelets | 134 | × 109/L | 150-450 |
| Automated Neutrophils | 2.02 | × 109/L | 1-8.5 |
| Automated Lymphocytes | 3 | × 109/L | 4-10.5 |
| Electrolytes | |||
| Sodium | 141 | mol/L | 136-145 |
| Potassium | 3.1 | mol/L | 3.5-5.1 |
| Chloride | 112 | mol/L | 98-107 |
| Urea | 1.8 | mol/L | 3.2-8.2 |
| Creatinine | 21 | mol/L | |
| Calcium | 2.07 | mol/L | 2.08-2.65 |
| Magnesium | 0.72 | mol/L | 0.70-1.0 |
| Phosphate | 1.68 | mol/L | 0.78-1.65 |
| Liver function test | |||
| Albumin | 30 | g/L | 40-47 |
| Alkaline Phosphatase (ALP) | 282 | U/L | 44-147 |
| Aspartate Amino Transferase (AST) | 37 | U/L | 13-40 |
| Alanine Amino Transferase (ALT) | 7 | U/L | 5-45 |
| Gamma-Glutamyl Transferase (GGT) | 15 | U/L | 8-60 |
| Total bilirubin | 4 | mol/L | 5-21 |
| Creatine Kinase (CK) | 36 | U/L | <250 |
| Lipid profile | |||
| Cholesterol | 4.58 | mol/L | 5.18-6.19 |
| Triglycerides | 1.85 | mol/L | <1.70 |
| Cardiac enzyme | |||
| Troponin-I | 0.01 | µg/L | 0.02-0.04 |
| Coagulation profile | |||
| Prothrombin Time (PT) | 18.9 | seconds | 11.7-15.3 |
| Activated Partial Thrombin Time (APTT) | 48.4 | seconds | 28.9-38.1 |
| International Normalized Ratio (INR) | 1.44 | ratio | 0.89-1.18 |
| Fibrinogen | 160 | mg/dL | 200-400 |
| D-Dimer | >20 | mg/L | 0-0.5 |
| Partial septic screen | |||
| Peripheral blood culture | No organism isolated after 5 days of incubation | -- | -- |
| Central blood culture | No organism isolated after 5 days of incubation | -- | -- |
| Urine culture | No growth | -- | -- |
| Lactic acid | 1.3 | mol/L | 0.50-2.20 |
| Venous blood gas | |||
| Ph | 7.18 | 7.35-7.45 | |
| Carbon dioxide (pCO2) | 36 | mmHg | 35-45 |
| Bicarbonate (HCO3-) | 13.6 | mol/L | 22-26 |
| Lactate | 7 | mol/L | <1 |
Table 1. Laboratory workup.
No increase in white blood cell count was noted. The patient had slightly disturbed electrolyte levels in the form of hypokalemia, hyperchloremia, and hyperphosphatemia. LFTs showed normal findings, except for mildly elevated Alkaline Phosphatase (ALP) levels. Coagulation profiles were abnormal, with high D-dimer levels. No organism growth was noted in the cultures that were sent at the hospital’s microbiology laboratory.
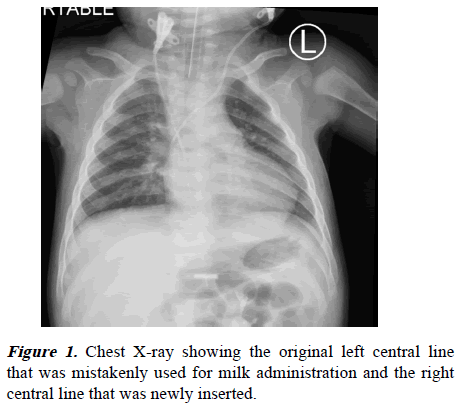
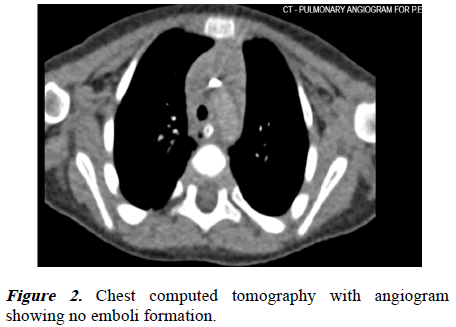
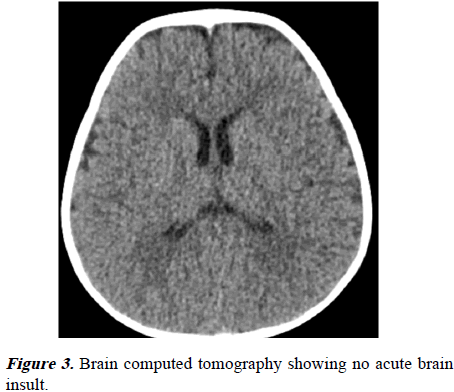
Radiological images were obtained, including chest X-ray, echocardiography, ECG, brain, and chest Computed Tomography (CT). The chest radiography (Figure 1) was clear of infiltrations, and the chest CT with angiogram (Figure 2) revealed no embolisms or pleural effusions. The ECG and echocardiography showed normal heart function with no right ventricular overload. The brain CT (Figure 3) showed no hypoxic injury.
Multiple disciplinary teams were involved, including vascular surgery, pediatric surgery, toxicology, infectious disease, hematology, and cardiology teams. Because the central line in the left jugular vein was the catheter through which enteral feed was administered, it was removed, and a new line was placed in the right internal jugular vein with satisfactory functions. The patient was started on meropenem 40 mg/kg/ dose Q8 hr and vancomycin 20 mg/kg/dose Q6 hr as per recommendations of the infectious disease specialists. One dose of anticoagulant was subcutaneously administered as per the hematologist’s recommendations as prophylaxis for thromboembolic events. The toxicology team recommended to keep patient Nil per Os (NPO) and to consider total parenteral nutrition if patient would be kept NPO for a duration more than 2 days.
The patient was hemodynamically stable 24 hours after the initial management. He was safely extubated to an oxygen facemask at a rate of 5 liter per minute before weaning to room air. After 48 hours in the PICU, the patient was transferred to the pediatric medical ward. No subsequent fatal outcomes were noted. Education about tube lines and their functions were thoroughly provided to his caregivers.
Discussion
Despite worldwide recognition of the issue, medication administration errors are a common preventable occurrence. Although intravenous milk administration is uncommon, it poses a considerable risk of patient injury with potentially preventable consequences [9].
A literature review conducted in 1972 revealed a case of unintentional intravenous breast milk administration in an infant who then developed respiratory distress and seizures; however, after supportive management, the patient did not experience any long-term adverse effects [9]. The composition, volume, and administration rate of enteral feeding could be related to the severity of clinical presentations; however, definitive conclusions cannot be drawn owing to the rarity of such reported cases [10].
The administration of a non-sterile fluid, such as enteral formulas or breast milk, through an intravenous route carries a high risk of fatality through sepsis, DIC, emboli, pulmonary embolism, renal or hepatic failure, seizures with possible neurological impairment, and anaphylactic reactions [9-11]. These adverse effects could be explained by the formation of micro embolisms from fat globules and insoluble fluid particles, in addition to the body’s immune reactions against foreign antigens [12,13].
Conclusion
Management of such cases with supportive therapy, broadspectrum antibiotics, and early involvement of multidisciplinary teams can prevent long-lasting adverse effects. In summary, intravenous administration of enteral formulas or breast milk continues to burden the healthcare system; although the incidence is low, it can cause many adverse effects. Thus, adequate staff and caregiver education may further decrease the occurrence of such cases. Labeling enteral feeding tubes and tracing the connected tube to its origin before administering medications or feeding may also reduce the chance of error.
Conflicts of Interest
Authors declare no conflict of interest.
Funding Statement
This research wasn’t funded by a specific company, hospital, or other facility.
Acknowledgements
The authors gratefully acknowledge the patient and their guardians for their participation in this study. We would like to thank Editage (www.editage.com) for English Language editing.