Current Pediatric Research
International Journal of Pediatrics
A clinico-epidemiological profile of neonates with birth asphyxia in SNCU and NICU of a tertiary care hospital with special reference to renal parameters and serum calcium levels in birth asphyxia.
Bidyut Kumar Khuntdar1, Arghya Kamal Roy2, Rajat Kumar Das3 and Ujjwal Pattanayak4*
1Department of Pediatrics, Tamralipto Government Medical College, Tamluk, West Bengal, India
2Department of Pediatrics, Midnapore Medical College, Midnapore, West Bengal, India
3Department of Pediatrics, KPC Medical College and Hospital, Kolkata, West Bengal, India
4Department of Community Medicine, KPC Medical College and Hospital, Kolkata, West Bengal, India
- Corresponding Author:
- Ujjwal Pattanayak
Department of Community Medicine,
KPC Medical College and Hospital,
Kolkata, West Bengal, India
E-mail: bkhuntdar1971@gmail.com
Received: 27 March, 2023, Manuscript No. AAJCP-23-89647; Editor assigned: 29 March, 2023, Pre QC No.AAJCP-23-89647(PQ); Reviewed: 07 April, 2023, QC No. AAJCP-23-89647; Revised: 17 April, 2023, Manuscript No. AAJCP-23-89647(R); Published: 27 April, 2023, DOI:10.35841/0971-9032.27.04.1185-1189
Background: Birth asphyxia accounts for around 23% of annual neonatal deaths in the developing world. Perinatal asphyxia or birth asphyxia as is more commonly used is one of the most common presentations of a neonate in Special Newborn Care Units (SNCUs) and Neonatal Intensive Care Units (NICUs) all around the world. Objective: The main objective of the study was to study electrolyte status and renal parameters in asphyxiated newborns of different severity in early neonatal period and to determine the occurrence of renal failure in asphyxiated neonates and to correlate severity with Apgar score and hypoxic ischemic encephalopathy grading of neonates. Methods: The present study was a prospective cross-sectional hospital based single centre study conducted in the Department of Paediatrics, Midnapore medical college, Paschim Medinipur, West Bengal, India. Terms, appropriate for gestational age babies born/admitted in SNCU and NICU with birth asphyxia between January, 2020 to June, 2021 were included in the study. Results: Our study showed that, in control group 1 (60%) were male and 6 (40%) were female. In case group 18 (51.4%) were male and 17 (48.6%) were female. Among 35 cases renal failure is seen in 68.57% and none of the controls had renal failure. Oliguria >0.5 ml/kg/hr was found in 14 (93.3%) patients in the control group, in case group it was found in 32 (91.4%) patients. The incidence of renal failure is highest with moderate asphyxia accounting for 41.67%. Conclusions: Perinatal asphyxia is one of the commonest clinical conditions encountered in the sick neonate. It affects almost all organ systems of the body. As the pre renal type of kidney injury is more common, these babies usually recover fully with fluid resuscitation in majority of cases.
Keywords
Birth asphyxia, Epidemiological profile, Neonates, Renal parameters, Serum calcium.
Introduction
WHO defines perinatal asphyxia as a failure to imitate or sustain breathing at birth [1]. The National Neonatal Perinatal Database (NNPD) 2000 used a similar definition for perinatal asphyxia. It defined moderate asphyxia as slow gasping breathing or an Apgar score of 4-6 at 1 minute of age and severe asphyxia was defined as no breathing or an Apgar score of 0-3 at 1 minute of age [2]. Overall the incidence of birth asphyxia ranges from 1%-1.5% at different centres and is related to the gestational age and birth weight of the baby. Perinatal asphyxia has far reaching consequences from a normal life to acute renal failure and cerebral palsy.
Perinatal asphyxia can cause hypoxic and ischaemic damage to all organs but kidneys, heart, lungs and liver but most serious involvement is that of the Central Nervous System (CNS). Sarnat and Levene staging are the two most widely used classifications of Hypoxic Ischemic Encephalopathy (HIE) which is the hallmark CNS manifestation of perinatal asphyxia [3,4]. Other classifications like Thompson’s staging are also used to grade severity of HIE.
Renal, CNS, lung and Cardiovascular (CVS) involvement were approximately 50%, 28%, and 25% each respectively [5,6]. According to the national neonatology forum of asphyxia has been defined as “gasping or ineffective breathing at one minute of life” [2,7]. Definitions based on Apgar scores may be useful as it can be used for formulating guidelines for post-asphyxial treatment of neonates. Apgar scores are also useful for predicting long term outcome in infants with perinatal asphyxia [8,9].
According to World Health Organization (WHO) latest estimates, approximately 4 million babies die each year before they reach the age of one month. Ninety-eight percent of these neonatal deaths take place in the developing countries. Perinatal asphyxia and birth injuries together contribute to almost 29% of this death [10].
According to NNPD 2003 data collected from 17 tertiary neonatal intensive care units in India, Apgar scores less than 7 at 1 minute of life, indicating moderate and severe asphyxia was found in 9% of all intramural deliveries. 2.5% babies continue to have Apgar scores below 7 at 5 minutes of age. Bag and mask ventilation was used in 4.5% infants. Less than 1% infants needed cardiac compressions and/or medications. 20% of all deaths in this age group could be attributed to perinatal asphyxia. Manifestations of HIE were seen in approximately 1.5% of all babies. Perinatal asphyxia was the commonest cause of still-births accounting for one-third of all such cases [11].
Perinatal hypoxia is one of the most common primary causes of neonatal mortality and morbidity in India, where a major proportion of deliveries are not institutional and most deliveries are conducted by untrained traditional birth attendants [7]. In India the incidence of perinatal hypoxia is as high as 8%-9% and it accounts for 28.8% of neonatal deaths and 45.1% of fresh still births [12-14].
Early recognition of renal failure is important in babies with HIE to facilitate appropriate fluid and electrolyte management as a stable electrical milieu is vital. Diagnosis of renal failure is difficult in this group as many clinical and biochemical parameters are unreliable in this age group. Hence this study was done to determine the incidence of kidney involvement, to get a rough estimate of incidence of acute renal failure in asphyxiated babies with renal involvement and correlate that with HIE grading with emphasis on early diagnosis of acute renal failure which may be of particular benefit for asphyxiated newborns at risk of developing renal failure.
Materials and Methods
Prospective cross-sectional hospital based single centre study was conducted in department of paediatrics, Midnapore medical college, Paschim Medinipur, West Bengal, India. Term, appropriate for gestational age babies born/admitted in SNCU and NICU of Midnapore medical college with birth Asphyxia between January, 2020 to June, 2021.
Inclusion criteria for cases
• Term, appropriate for gestational age, newborns with history of birth Asphyxia.
• Evidence of neurologic abnormalities suggestive of hypoxic ischemic encephalopathy.
Exclusion criteria for cases
• Babies with septicemia, respiratory distress syndrome.
• Neonates who have received aminoglycoside antibiotics and aminophylline.
• Congenital anomalies of the kidneys and/or urinary tract.
• Preterm babies with birth asphyxia
Method of data collection
35 term newborn, appropriate for gestational age babies admitted to SNCU and NICU of Midnapore medical college and hospital with birth asphyxia (cases) and 15 healthy neonates (controls) are included in the study. Informed written consent was taken from the parents of the baby after they had signed the patient information sheet. Antenatal and intranatal history was taken from hospital records. Detailed clinical examination was done. Newborns identified as appropriate for gestational age by applying Ballard’s score and by percentile chart. APGAR score was done to identify asphyxiated neonates and those with Hypoxic ischemic encephalopathy are classified by Sarnat staging.
Relevant antenatal history, gestational age of baby, weight, findings on physical examination and systemic examination were recorded on predesigned proforma. Renal function parameters–urine output, urine analysis, and urine sodium and creatinine and serum electrolytes with serum calcium were monitored initially within 24 hrs of birth. At 48 hrs and 72 hrs in addition, blood urea and creatinine were measured.
Differentiation between pre renal and intrinsic renal failure was done with the help of renal indices like Fractional Excretion of Sodium (FENa) and renal failure index. Ultrasonography was done to rule out congenital renal anomalies. On the basis of Apgar score at 5 minutes the asphyxiated babies are grouped into mild (score of 6-7), moderate (score of 4-5) and severe asphyxia (score of 3 or less).
Statistical analysis
For statistical analysis data were entered into a Microsoft excel spreadsheet and then analyzed by SPSS (version 27.0; SPSS Inc., Chicago, IL, USA). tabulation, proportion, percentage, mean standard deviation and different diagrams like bar diagram and pie chart will were used
Results
Prospective cross-sectional hospital based single centre study was conducted in department of paediatrics, Midnapore medical college, Paschim Medinipur, West Bengal, India. Term, appropriate for gestational age babies born/admitted in SNCU and NICU of Midnapore medical college with birth asphyxia between January, 2020 to June, 2021. 35 term newborn, appropriate for gestational age babies admitted to SNCU and NICU of Midnapore medical college and hospital with birth asphyxia (cases) and 15 healthy neonates (controls) are included in the study in all the cases, thorough history taking and clinical examination was done after taking proper consent from the parents. Data thus obtained was noted in the proforma. Results thus obtained were analysed and expressed in tables
In control group 1 (60%) were male and 6 (40%) were female. In case group 18 (51.4%) were male and 17 (48.6%) were female. Among 35 cases renal failure is seen in 68.57% and none of the controls had renal failure. Oliguria >0.5 ml/kg/hr was found in 14 (93.3%) patients in the control group, in case group it was found in 32 (91.4%) patients (Table 1).
| Gender | Control (n=15) | Case (n-35) | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| Male | 9 | 60 | 18 | 51.4 |
| Female | 6 | 40 | 17 | 48.6 |
| Incidence of renal failure | ||||
| Yes | 0 | 0 | 24 | 68.57 |
| No | 0 | 0 | 11 | 31.43 |
| Oliguria | ||||
| <0.5 ml/kg/hr | 1 | 6.7 | 3 | 8.6 |
| >0.5 ml/kg/hr | 14 | 93.3 | 32 | 91.4 |
Table 1. Distribution of participants according to gender, incidence of renal failure and oliguria.
Our study population included majority with of neonates with mild and moderate birth asphyxia accounting for 40% each with severe birth asphyxia accounting for 20%. Majority of cases were in HIE stage II accounting for 45.71% with stage I, stage III and no HIE were 25.71%, 17.14% and 11.42% respectively. The incidence of renal failure is highest with moderate asphyxia accounting for 41.67% (Table 2).
| Case (n-35) | ||
|---|---|---|
| Number | Percentage | |
| Severe | 7 | 20 |
| Moderate | 14 | 40 |
| Mild | 14 | 40 |
| HIE Stage | ||
| I | 9 | 25.71 |
| II | 16 | 45.71 |
| III | 6 | 17.14 |
| No | 4 | 11.42 |
| Total | 35 | 100 |
| Renal Failure with severity of asphyxia | ||
| Mild | 7 | 5 |
| Moderate | 10 | 3 |
| Severe | 7 | 3 |
| Total | 24 | 11 |
Table 2. Distribution of cases according to severity of asphyxia, to HIE staging and renal failure with severity of asphyxia (n=35).
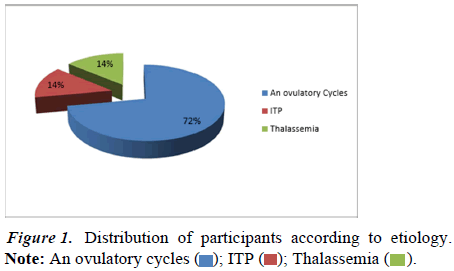
From the above figure we conclude that 72 (72.0%) patients had ovulatory cycles, 14 (14.0%) patients had ITP and 14 (14.0%) patients had thalassemia. The value of z is 8.284. The value of p is <0.00001. The result is significant at p<0.05 (Figure 1).
In this study serum sodium levels with severity of birth asphyxia among study population as compared to the control group and the difference between the two groups was statistically significant <24 hrs. Serum calcium levels with severity of birth asphyxia among study population as compared to the control group and the difference between the two groups was not statistically significant. Variation of Serum urea levels with severity of birth asphyxia was also not statistically significant (Table 3).
| Concentration of serum sodium | Group | N | Mean ± SD (mmol/L) | Significance |
|---|---|---|---|---|
| <24 Hours | Severe | 7 | 129.85 ± 2.45 | <0.001 |
| Moderate | 14 | 132.26 ± 3.93 | ||
| Mild | 14 | 136.23 ± 2.35 | ||
| 48 hours | Severe | 7 | 130.24 ±1.46 | 0.346 |
| Moderate | 14 | 133.12 ± 4.66 | ||
| Mild | 14 | 134.86 ± 2.96 | ||
| 72 hours | Severe | 7 | 129.24 ± 3.64 | 0.023 |
| Moderate | 14 | 132.36 ± 5.88 | ||
| Mild | 14 | 135.56 ± 2.94 | ||
| Concentration of serum calcium | ||||
| <24 Hours | Severe | 7 | 9.10 ± 1.30 | 0.248 |
| Moderate | 14 | 8.66 ± 1.22 | ||
| Mild | 14 | 9.37 ± 0.78 | ||
| 48 hours | Severe | 7 | 7.96 ± 0.92 | 0.006 |
| Moderate | 14 | 7.88 ± 0.90 | ||
| Mild | 14 | 8.86 ± 0.65 | ||
| 72 hours | Severe | 7 | 7.80 ± 0.86 | 0.023 |
| Moderate | 14 | 8.23 ± 1.22 | ||
| Mild | 14 | 8.92 ± 0.66 | ||
| Concentration of serum urea | ||||
| 48 hours | Severe | 7 | 28.26 ± 11.25 | 0.659 |
| Moderate | 14 | 34.62 ± 14.32 | ||
| Mild | 14 | 32.25 ± 17.84 | ||
| 72 hours | Severe | 7 | 36.14 ± 19.64 | 0.423 |
| Moderate | 14 | 35.12 ± 13.16 | ||
| Mild | 14 | 30.21 ± 15.36 | ||
Table 3. Serum sodium levels, serum calcium levels, variation of serum urea levels and urine sodium levels with severity of birth asphyxia (n=35).
Variation of urine sodium levels with severity of birth asphyxia was also not statistically significant. In our study we found that urine creatinine was found to be higher among the cases than the controls and the difference between the groups were statistically significant. The neonates who had mild asphyxia had lower creatinine excretion as compared to those neonates with severe asphyxia with statistical difference between the groups.
Variation of fractional excretion of sodium with severity of birth asphyxia was also not statistically significant. The renal failure index was higher among cases as compared to controls which was statistically significant and correlated with the severity of asphyxia and with HIE stage (Table 4).
| Urine sodium | Group | N | Mean ± SD (mmol/L) | Significance |
|---|---|---|---|---|
| Day 2 | Severe | 7 | 43.14 ± 9.62 | 0.042 |
| Moderate | 14 | 40.42 ± 18.26 | ||
| Mild | 14 | 29.40 ± 11.34 | ||
| Day 3 | Severe | 7 | 47.57 ± 11.12 | 0.047 |
| Moderate | 14 | 39.54 ± 19.38 | ||
| Mild | 14 | 29.98 ± 14.82 | ||
| Urine Creatinine | ||||
| Day 2 | Severe | 7 | 17.21 ± 2.78 | 0.036 |
| Moderate | 14 | 24.12 ± 9.26 | ||
| Mild | 14 | 26.22 ± 6.36 | ||
| Day 3 | Severe | 7 | 16.88 ± 2.08 | 0.022 |
| Moderate | 14 | 23.98 ± 8.76 | ||
| Mild | 14 | 26.33 ± 6.66 | ||
| FeNa | ||||
| Day 2 | Severe | 7 | 3.66 ± 1.44 | 0.046 |
| Moderate | 14 | 2.66 ± 2.3 | ||
| Mild | 14 | 1.64 ± 1.80 | ||
| Day 3 | Severe | 7 | 3.84 ± 1.01 | 0.02 |
| Moderate | 14 | 2.12 ± 1.46 | ||
| Mild | 14 | 1.14 ± 1.56 | ||
| RFI | ||||
| Day 2 | Severe | 7 | 4.21 ± 2.02 | 0.093 |
| Moderate | 14 | 3.34 ± 2.3 | ||
| Mild | 14 | 1.98 ± 2.22 | ||
| Day 3 | Severe | 7 | 4.36 ± 2.03 | 0.018 |
| Moderate | 14 | 2.81 ± 2.36 | ||
| Mild | 14 | 1.88 ± 1.64 | ||
Table 4. Urine sodium levels, urine creatinine levels, fractional excretion of sodium and variation of renal failure index with severity of birth asphyxia.
Discussion
Perinatal asphyxia is an insult occurring to the fetus due to a lack of blood flow or gas exchange to or from the fetus occurring in the period immediately before, during or after the process of birth. It can result in profound neurological and systemic sequelae. When placental (prenatal) or pulmonary (immediate post-natal) gas exchange is compromised or ceases altogether, there is partial (hypoxia) or complete (anoxia) lack of oxygen to the vital organs. This results in progressive hypoxemia and hypercapnia. If the hypoxemia is severe enough, the tissues and vital organs (muscle, liver, heart, and ultimately the brain) will develop an oxygen debt. Anaerobic glycolysis and lactic acidosis will result. Neonatal hypoxicischemic encephalopathy refers specifically to the neurologic sequelae of perinatal asphyxia [15].
Our study is a case control study done among neonates admitted in SNCU and NICU of our institution. In this study 35 cases of varying severity and grades of perinatal asphyxia and 15 controls, who were healthy neonates without any disease, were taken. All relevant information like perinatal history, gestational age, birth weight and clinical examination findings were recorded in a predesigned and pretested proforma. Sarnat staging was used to grade the cases according to their severity. We also analysed the serum electrolytes at 24, 48 and 72 hours of life. In addition, analysed the serum urea and creatinine values at the same intervals.
Monitoring of the renal system was done with urine output measurement, urinalysis which included urinary creatinine and sodium. Fractional excretion of sodium (FeNa) and Renal Failure Index (RFI) was calculated. In our study 51.4% of the cases were male. A study conducted by Macdonald et al. among 38405 consecutive deliveries to identify birth asphyxia on the basis of requirement of positive pressure ventilation found that 54% of asphyxiated babies were make and 46% female [16].
The mean serum urea values at 48 and 72 hours of life among cases was 34.65 ± 16.28 mg/dl and 33.84 ± 16.56 mg/dl respectively. This was significantly higher and statistically significant as compared to the values among controls. Jayashree, et al. showed in their study that the mean blood urea level was 94 ± 34.7 mg/dl among the cases. This was significantly higher compared to controls that had a mean value of 25.6 mg/dl. Majority of cases fell under HIE 3 category (55.5%) [7].
The mean Serum creatinine value among cases was 1.31 ± 0.64 mg/dl as compared to controls who had values of 0.67 ± 0.43 mg/dl at the same time frame. This is statistically significant. Gupta, et al. conducted a similar study and found that the mean serum creatinine value was 1.08+0.49 among the 70 asphyxiated neonates they studied as compared to controls had 0.88+0.26 mg/dl [17].
The mean sodium value among cases in our study was 132.78 ± 4.07 mmol/l as compared to controls in whom the mean value was 138.04 ± 2.08 mmol/l. P Basu and coworkers studied the electrolyte status in asphyxiated neonates and found that mean serum sodium levels were significantly lower in asphyxiated babies (122+6.0 mEq/L) as compared to controls (138.8+2.7 mEq/L) with p value of <0.001.18.
The mean potassium value among cases in our study was 5.22± 0.54 mmol/l as compared to controls in which the mean value was 4.75 ± 0.93 mmol/l. A study done by BD Gupta et al found that the serum potassium levels were slightly higher in cases as compared to the controls [17]. The mean calcium value among cases in our study was 8.56 ± 1.01 mg/dl as compared to controls in which the mean value was 9.20 ± 0.23 mg/dl.
Basu, et al. coworkers conducted a similar study to assess electrolye status in asphyxiated neonates. They found that serum calcium is significantly lower in asphyxiated babies as compared to healthy controls. This is statistically significant [18]. The mean FeNa value among the cases was 2.32% ± 1.96% as compared to controls had 0.84 ± 0.33%. A study done by Misra, et al. found that fractional excretion of sodium (FENa+) among asphyxiated neonates was 3.25%+1.25% as compared to 0.134%+0.007% in controls [2].
Conclusion
Perinatal asphyxia is one of the commonest clinical conditions encountered in the sick neonate. It affects almost all organ systems of the body. Of the various types of renal failure seen in neonates, non oliguric renal failure is usually the most common type. As such only urine output monitoring is insufficient in these babies and different renal parameters should also be taken into consideration. Serial monitoring of serum urea, creatinine, and electrolytes should be done in these babies as it helps in prevention, early detection and subsequent management of renal failure.
Biochemical parameters in urine like urinary sodium and creatinine should also be monitored in asphyxiated babies with renal involvement as they are essential for calculation of renal indices like fractional excretion of sodium and Renal failure index which helps to understand whether the renal failure is pre renal or intrinsic. Acute renal failure in birth asphyxia shows a strong positive correlation with the stage of HIE.
Ethical Clearance
The study was conducted only after obtaining written approval from the institutional ethics committee vide memo no MMC/ IEC-2020/309, dated 11.02.2020. Written informed consent was taken from the parents.
Acknowledgements
Authors would like to acknowledge the patients who participated in this research study.
Funding
No funding sources.
Conflict of Interest
None declared.
Ethical Approval
The study was approved by the institutional ethics committee.
 ; ITP
; ITP  Thalassemia
Thalassemia  .
.